CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
AS3500000
-
CHEMICAL NAME :
-
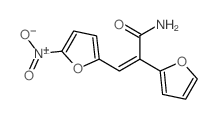
Acrylamide, 2-(2-furyl)-3-(5-nitro-2-furyl)-
-
CAS REGISTRY NUMBER :
-
3688-53-7
-
LAST UPDATED :
-
199606
-
DATA ITEMS CITED :
-
88
-
MOLECULAR FORMULA :
-
C11-H8-N2-O5
-
MOLECULAR WEIGHT :
-
248.21
-
WISWESSER LINE NOTATION :
-
T5OJ BYVZU1- BT5OJ ENW
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1554 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
221 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1740 mg/kg/29D-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - hepatic microsomal mixed oxidase (dealkylation, hydroxylation, etc.)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2500 mg/kg/21D-I
-
TOXIC EFFECTS :
-
Liver - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - hepatic microsomal mixed oxidase (dealkylation, hydroxylation, etc.) Biochemical - Metabolism (Intermediary) - other proteins
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
52 gm/kg/40W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
156 gm/kg/44W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Lungs, Thorax, or Respiration - tumors Gastrointestinal - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
150 mg/kg female 13-17 day(s) after conception
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Reproductive - Tumorigenic effects - transplacental tumorigenesis Lungs, Thorax, or Respiration - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
63 gm/kg/94W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Gastrointestinal - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
25 gm/kg/78W-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Gastrointestinal - tumors Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
211 gm/kg/63W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Gastrointestinal - tumors Blood - leukemia
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
158 gm/kg/47W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Gastrointestinal - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
42 gm/kg/63W-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Gastrointestinal - tumors Blood - leukemia
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
127 gm/kg/94W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Gastrointestinal - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
116 gm/kg/80W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Gastrointestinal - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
32400 mg/kg/72W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Gastrointestinal - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6 gm/kg
-
SEX/DURATION :
-
female 1-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 1-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6600 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
720 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5750 mg/kg
-
SEX/DURATION :
-
male 5 week(s) pre-mating female 5 week(s) pre-mating - 3 week(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - biochemical and metabolic Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
28750 mg/kg
-
SEX/DURATION :
-
multigenerations
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 13-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material)
-
TYPE OF TEST :
-
Specific locus test
-
TYPE OF TEST :
-
Sex chromosome loss and nondisjunction
-
TYPE OF TEST :
-
Sperm Morphology
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Morphological transformation
-
TYPE OF TEST :
-
Cytogenetic analysis
MUTATION DATA
-
TYPE OF TEST :
-
Host-mediated assay
-
TEST SYSTEM :
-
Rodent - hamster Fibroblast
-
DOSE/DURATION :
-
50 mg/kg
-
REFERENCE :
-
GMCRDC Gann Monograph on Cancer Research. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No. 11- 1971- Volume(issue)/page/year: 27,73,1981 *** REVIEWS *** IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 31,47,1983 IARC Cancer Review:Human No Adequate Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 31,47,1983 IARC Cancer Review:Group 2B IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,56,1987 TOXICOLOGY REVIEW CCSUDL Carcinogenesis--A Comprehensive Survey. (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) V.1- 1976- Volume(issue)/page/year: 4,171,1978
|