jolkinolide E
Modify Date: 2025-08-25 12:42:53
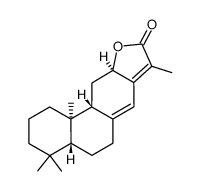
jolkinolide E structure
|
Common Name | jolkinolide E | ||
|---|---|---|---|---|
| CAS Number | 54494-34-7 | Molecular Weight | 300.43500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H28O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of jolkinolide EJolkinolide E is a casbane diterpenoid from the roots of Euphorbia rapulum. Jolkinolide E shows weak selective activity against HepG2, MCF-7, and C6 cell lines[1]. |
| Name | ent-abieta-8(14),13(15)-dien-16,12-olide |
|---|---|
| Synonym | More Synonyms |
| Description | Jolkinolide E is a casbane diterpenoid from the roots of Euphorbia rapulum. Jolkinolide E shows weak selective activity against HepG2, MCF-7, and C6 cell lines[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C20H28O2 |
|---|---|
| Molecular Weight | 300.43500 |
| Exact Mass | 300.20900 |
| PSA | 26.30000 |
| LogP | 4.80100 |
| InChIKey | ZXEVPUOHSXARBR-UHFFFAOYSA-N |
| SMILES | CC1=C2C=C3CCC4C(C)(C)CCCC4(C)C3CC2OC1=O |
| jolkinolide E |
| helioscopoindolide G |
| (4aR,10aR,11aR,11bR)-4,4,8,11b-Tetramethyl-2,3,4,4a,5,6,10a,11,11a,11b-decahydro-1H-10-oxa-cyclopenta[b]phenanthren-9-one |

