Etifoxine hydrochloride
Modify Date: 2025-08-20 18:48:24
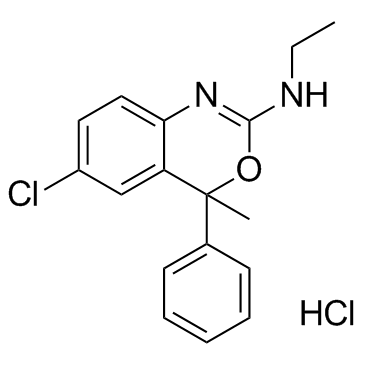
Etifoxine hydrochloride structure
|
Common Name | Etifoxine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 56776-32-0 | Molecular Weight | 337.244 | |
| Density | 1.2g/cm3 | Boiling Point | 421.2ºC at 760mmHg | |
| Molecular Formula | C17H18Cl2N2O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 208.5ºC | |
Use of Etifoxine hydrochlorideEtifoxine Hcl(HOE 36-801) is potentiator of GABAA receptor function in cultured neurons. Etifoxine preferentially acts on β2 or β3 subunit-containing GABAA receptors. IC50 value:Target: GABAA receptorEtifoxine exhibits anxiolytic activity in rodents and humans with no sedative, myorelaxant or mnesic side effects. Etifoxine acts as a ligand of the translocator protein (TSPO); promotes axonal regeneration. |
| Name | Etifoxine Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Etifoxine Hcl(HOE 36-801) is potentiator of GABAA receptor function in cultured neurons. Etifoxine preferentially acts on β2 or β3 subunit-containing GABAA receptors. IC50 value:Target: GABAA receptorEtifoxine exhibits anxiolytic activity in rodents and humans with no sedative, myorelaxant or mnesic side effects. Etifoxine acts as a ligand of the translocator protein (TSPO); promotes axonal regeneration. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2g/cm3 |
|---|---|
| Boiling Point | 421.2ºC at 760mmHg |
| Molecular Formula | C17H18Cl2N2O |
| Molecular Weight | 337.244 |
| Flash Point | 208.5ºC |
| Exact Mass | 336.079620 |
| PSA | 33.62000 |
| LogP | 4.85920 |
| InChIKey | SCBJXEBIMVRTJE-UHFFFAOYSA-N |
| SMILES | CCN=C1Nc2ccc(Cl)cc2C(C)(c2ccccc2)O1.Cl |
| Storage condition | 2-8℃ |
| RIDADR | NONH for all modes of transport |
|---|
| 6-Chloro-N-ethyl-4-methyl-4-phenyl-4H-3,1-benzoxazin-2-amine hydrochloride (1:1) |
| 4H-3,1-Benzoxazin-2-amine, 6-chloro-N-ethyl-4-methyl-4-phenyl-, hydrochloride (1:1) |
| 6-chloro-N-ethyl-4-methyl-4-phenyl-3,1-benzoxazin-2-amine,hydrochloride |
| 4H-3,1-Benzoxazin-2-amine, 6-chloro-N-ethyl-4-methyl-4-phenyl-, monohydrochloride |
| Etifoxine (hydrochloride) |