Isochlorogenic acid C(4,5)
Modify Date: 2024-01-03 11:53:29
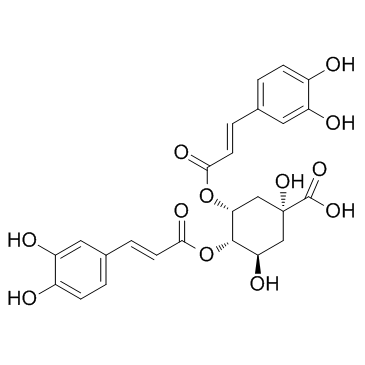
Isochlorogenic acid C(4,5) structure
|
Common Name | Isochlorogenic acid C(4,5) | ||
|---|---|---|---|---|
| CAS Number | 57378-72-0 | Molecular Weight | 516.451 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 810.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C25H24O12 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 274.9±27.8 °C | |
Use of Isochlorogenic acid C(4,5)4,5-Dicaffeoylquinic acid ( Isochlorogenic acid C) possesses potent hepatoprotective and anti-HBV effects.IC50 value:Target: Anti-hepatitis natural produce.In vitro: To study anti-hepatitis effect of isochlorogenic acid C, anti-apoptotic and anti-injury properties of test compound were evaluated. The results showed that test compound at concentrations of 10 to 100 μg/ml significantly reduced the caspase-3 and transformed growth factor β1 (TGFβ1) levels of the D-GalN-challenged hepatocytes. Also, test compound improved markedly cell viability of the D-GalN-injured hepatocytes and produced a maximum protection rate of 47.28% at a concentration of 100 μg/ml. Furthermore, test compound significantly inhibited productions of HBsAg and HBeAg. Its maximum inhibitory rates on the HBsAg and HBeAg expressions were 86.93 and 59.79%, respectively. In addition, test compound significantly induced the HO-1 expression of HepG2.2.15 cells [1]. In vivo: |
| Name | (1R,3R,4S,5R)-1,5-Dihydroxy-3,4-bis[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1-cyclohexanecarboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 4,5-Dicaffeoylquinic acid ( Isochlorogenic acid C) possesses potent hepatoprotective and anti-HBV effects.IC50 value:Target: Anti-hepatitis natural produce.In vitro: To study anti-hepatitis effect of isochlorogenic acid C, anti-apoptotic and anti-injury properties of test compound were evaluated. The results showed that test compound at concentrations of 10 to 100 μg/ml significantly reduced the caspase-3 and transformed growth factor β1 (TGFβ1) levels of the D-GalN-challenged hepatocytes. Also, test compound improved markedly cell viability of the D-GalN-injured hepatocytes and produced a maximum protection rate of 47.28% at a concentration of 100 μg/ml. Furthermore, test compound significantly inhibited productions of HBsAg and HBeAg. Its maximum inhibitory rates on the HBsAg and HBeAg expressions were 86.93 and 59.79%, respectively. In addition, test compound significantly induced the HO-1 expression of HepG2.2.15 cells [1]. In vivo: |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 810.8±65.0 °C at 760 mmHg |
| Molecular Formula | C25H24O12 |
| Molecular Weight | 516.451 |
| Flash Point | 274.9±27.8 °C |
| Exact Mass | 516.126770 |
| PSA | 211.28000 |
| LogP | 0.89 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.719 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
| 4,5-di-O-trans-caffeoyl-D-quinic acid |
| (1R,3R,4S,5R)-3,4-Bis{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,5-dihydroxycyclohexanecarboxylic acid |
| 4,5-Dinitrophenanthren |
| Cyclohexanecarboxylic acid, 3,4-bis[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy]-1,5-dihydroxy-, (1R,3R,4S,5R)- |
| (1R,3R,4S,5R)-3,4-Bis{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}-1,5-dihydroxycyclohexanecarboxylic acid |
| Isochlorogenic acid C |
| (1S,3R,4S,5R)-4-((E)-4-(3,4-Dihydroxyphenyl)-2-oxobut-3-en-1-yl)-3-(((E)-3-(3,4-dihydroxyphenyl)acryloyl)oxy)-5-hydroxy-1-methylcyclohexanecarboxylic acid |
| N1748 |
| 4,5-di-O-caffeoylquinic acid |
| 4,5-di-O-trans-caffeoylquininc acid |
| Phenanthrene,4,5-dinitro |
| 4,5-Dicaffeoylquinic acid |

