Guvacine hydrochloride
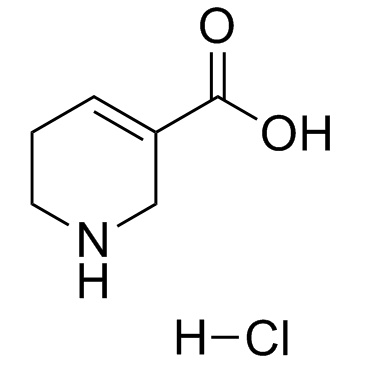
Guvacine hydrochloride structure
|
Common Name | Guvacine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 6027-91-4 | Molecular Weight | 163.60200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H10ClNO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Guvacine hydrochlorideGuvacine hydrochloride is an alkaloid from the nut of Areca catechu, acts as an inhibitor of GABA transporter, and dispalys modest selectivity for cloned GABA transporters with IC50s of 14 μM (human GAT-1), 39 μM (rat GAT-1), 58 μM (rat GAT-2), 119 μM (human GAT-3), 378 μM (rat GAT-3), and 1870 μM (human BGT-3). |
| Name | 1,2,5,6-Tetrahydro-pyridine-3-carboxylic acid hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Guvacine hydrochloride is an alkaloid from the nut of Areca catechu, acts as an inhibitor of GABA transporter, and dispalys modest selectivity for cloned GABA transporters with IC50s of 14 μM (human GAT-1), 39 μM (rat GAT-1), 58 μM (rat GAT-2), 119 μM (human GAT-3), 378 μM (rat GAT-3), and 1870 μM (human BGT-3). |
|---|---|
| Related Catalog | |
| Target |
IC50: 14 μM (human GAT-1), 39 μM (rat GAT-1), 58 μM (rat GAT-2), 119 μM (human GAT-3), 378 μM (rat GAT-3), 1870 μM (human BGT-3)[1] |
| In Vitro | Guvacine hydrochloride is a potent inhibitor of GABA transporter, dispalys modest selectivity forcloned GABA transporters with IC50s of 14 μM (human GAT-1), 39 μM (rat GAT-1), 58 μM (rat GAT-2), 119 μM (human GAT-3), 378 μM (rat GAT-3), and 1870 μM (human BGT-3). Guvacine has low affinity at hBGT-1 (IC50 >1 mM)[1]. Guvacine hydrochloride is a potent inhibitor of GABA uptake, but does not inhibit sodium-independent GABA binding, and is weak or inactive as a GABA receptor agonist[2]. Guvacine inhibits the uptake GABA and β-alanine with IC50s of 23 ± 2 μM, 66 ± 11 μM in the Cat spinal cord, and 8 ± 1 μM, 123 ± 28 μM in the rat cerebral cortex, respectively[3]. |
| Cell Assay | Cells grown in 24-well plates are washed 3 × with Hepes-buffered saline (HBS, in mM: NaC1, 150; Hepes, 20; CaCl2, 1; glucose, 10; KC1, 5; MgCl2, 1; pH 7.4) and allowed to equilibrate on a 37°C slide warmer. After 10 min the medium is removed and unlabeled drugs (Guvacine , etc.) in HBS are added (450 μL/well). Transport is initiated by adding 50 μL per well of a concentrated solution of [3H]GABA in HBS (final concentration = 50 nM). Non-specific uptake is defined in parallel wells with 1 mM unlabeled GABA, and is subtracted from total uptake to yield specific uptake; all data represent specific uptake. Plates are incubated at 37°C for 10 min, then washed rapidly 3 × with ice-cold HBS, using a 24-position plate washer. Cells are solubilized with 0.05% sodium deoxycholate/0.1 N NaOH (0.25 mL/well), an aliquot neutralized with 1 N HC1, and radioactivity is determined by scintillation counting. Protein is quantified in an aliquot of the solubilized cells using a BIO-RAD protein assay kit[1]. |
| References |
| Molecular Formula | C6H10ClNO2 |
|---|---|
| Molecular Weight | 163.60200 |
| Exact Mass | 163.04000 |
| PSA | 49.33000 |
| LogP | 1.12150 |
| Storage condition | 2-8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933399090 |
|
~% 
Guvacine hydroc... CAS#:6027-91-4 |
| Literature: US2009/258883 A1, ; Page/Page column 12 ; US 20090258883 A1 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Structural insights into thyroid hormone transport mechanisms of the L-type amino acid transporter 2.
Mol. Endocrinol. 29 , 933-42, (2015) Thyroid hormones (THs) are transported across cell membranes by different transmembrane transporter proteins. In previous studies, we showed marked 3,3'-diiodothyronine (3,3'-T2) but moderate T3 uptak... |
|
|
Gabapentin attenuates neuropathic pain and improves nerve myelination after chronic sciatic constriction in rats.
Neurosci. Lett. 607 , 52-8, (2015) Gabapentin (GBP) is an anti-convulsive drug often used as analgesic to control neuropathic pain. This study aimed at evaluating oral GBP treatment (30, 60, 120 mg/kg, 60 min prior to chronic constrict... |
|
|
Development of a SPE-HPLC-MS/MS method for the determination of most prescribed pharmaceuticals and related metabolites in urban sewage samples.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 990 , 23-30, (2015) Based on regional prescription data several pharmaceuticals with variable amounts of prescription and corresponding metabolites were selected and analyzed in influent and effluent samples of the sewag... |
| GUVACINE HYDROCHLORIDE &3-Pyridinecarboxylic acid,1,2,5,6-tetrahydro-,hydrochloride |
| 1,2,5,6-Tetrahydro-pyridin-3-carbonsaeure,Hydrochlorid |
| 1,2,5,6-Tetrahydro-3-pyridinecarboxylic Acid Hydrochloride |
| Dilazep dihydrochloride |
| Guvacine hydrochloride |
| 1,2,5,6-Tetrahydro |
| GABA UPTAK |
| 1,2,5,6-Tetrahydropyridine-3-carboxylic acid hydrochloride |
| 1,2,5,6-tetrahydro-3-pyridinecarboxylic acid,hydrochloride salt |
 CAS#:86447-11-2
CAS#:86447-11-2
