N-alpha-Acetyl-L-ornithine
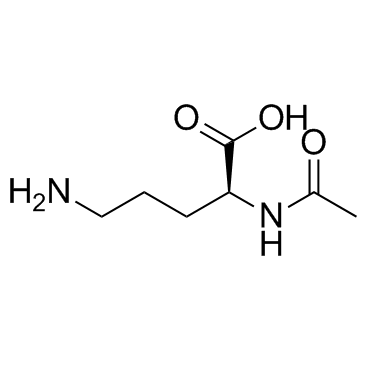
N-alpha-Acetyl-L-ornithine structure
|
Common Name | N-alpha-Acetyl-L-ornithine | ||
|---|---|---|---|---|
| CAS Number | 6205-08-9 | Molecular Weight | 174.19800 | |
| Density | 1.171g/cm3 | Boiling Point | 436.2ºC at 760 mmHg | |
| Molecular Formula | C7H14N2O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 217.6ºC | |
Use of N-alpha-Acetyl-L-ornithineN-Acetylornithine is an intermediate in the enzymatic biosynthesis of the amino acid L-arginine from L-glutamate. |
| Name | N2-acetyl-L-ornithine |
|---|---|
| Synonym | More Synonyms |
| Description | N-Acetylornithine is an intermediate in the enzymatic biosynthesis of the amino acid L-arginine from L-glutamate. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| Density | 1.171g/cm3 |
|---|---|
| Boiling Point | 436.2ºC at 760 mmHg |
| Molecular Formula | C7H14N2O3 |
| Molecular Weight | 174.19800 |
| Flash Point | 217.6ºC |
| Exact Mass | 174.10000 |
| PSA | 92.42000 |
| LogP | 0.40580 |
| Appearance of Characters | Solid |
| Index of Refraction | 1.492 |
| InChIKey | JRLGPAXAGHMNOL-LURJTMIESA-N |
| SMILES | CC(=O)NC(CCCN)C(=O)O |
| Storage condition | −20°C |
| Water Solubility | Freely soluble (880 g/L) (25 ºC) |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924199090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Metabolomic profiling of serum in the progression of Alzheimer's disease by capillary electrophoresis-mass spectrometry.
Electrophoresis 35(23) , 3321-30, (2014) There is high interest in the discovery of early diagnostic biomarkers of Alzheimer's disease, for which metabolomics exhibits a great potential. In this work, a metabolomic approach based on ultrafil... |
|
|
Inhibitors of N(alpha)-acetyl-L-ornithine deacetylase: synthesis, characterization and analysis of their inhibitory potency.
Amino Acids 38 , 1155-1164, (2010) A series of N (alpha)-acyl (alkyl)- and N (alpha)-alkoxycarbonyl-derivatives of L- and D-ornithine were prepared, characterized, and analyzed for their potency toward the bacterial enzyme N (alpha)-ac... |
|
|
Model-driven discovery of underground metabolic functions in Escherichia coli.
Proc. Natl. Acad. Sci. U. S. A. 112(3) , 929-34, (2015) Enzyme promiscuity toward substrates has been discussed in evolutionary terms as providing the flexibility to adapt to novel environments. In the present work, we describe an approach toward exploring... |
| N(2)-acetyl-L-ornithine |
| N-acetylornithine |
| ACETYL-L-ORNITHINE |
| Ornithine,N2-acetyl |
| AOR |
| (2S)-2-acetamido-5-aminopentanoic acid |
| N|A-Acetyl-L-ornithine |
| N-α-acetyl-l-ornithine |
| N-alpha-Acetyl-L-ornithine |
 CAS#:70-26-8
CAS#:70-26-8
