PD 168077 maleate
Modify Date: 2024-01-14 15:16:13
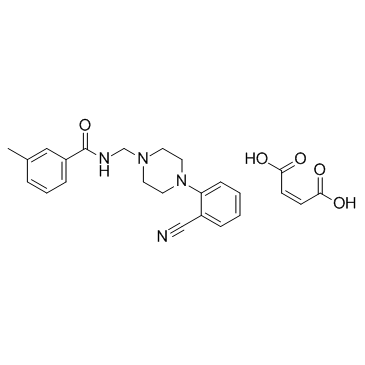
PD 168077 maleate structure
|
Common Name | PD 168077 maleate | ||
|---|---|---|---|---|
| CAS Number | 630117-19-0 | Molecular Weight | 450.487 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C24H26N4O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of PD 168077 maleatePD-168077 maleate is a selective dopamine D4 receptor agonist, with a Ki of 9 nM. |
| Name | N-{[4-(2-Cyanophenyl)-1-piperazinyl]methyl}-3-methylbenzamide (2Z)-2-butenedioate (1:1) |
|---|---|
| Synonym | More Synonyms |
| Description | PD-168077 maleate is a selective dopamine D4 receptor agonist, with a Ki of 9 nM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 9 nM (Dopamine D4 receptor)[1]. |
| In Vitro | PD-168077 is one of the first agents to be identified as putative selective D4 agonists. It shows >100-fold selectivity over other members of the D2-like receptor family and over their D1-like counterparts; PD-168077 shows a 20-fold selectivity over α1, and α2, a 45-fold selectivity over 5-HT1A, and a 460-fold selectivity over 5-HT2A receptors; PD-168077 evidences intrinsic activity at the D4 receptor in terms of quinpirole-like inhibition of forskolin-stimulated cAMP accumulation or stimulation of [3H]thymidine uptake in CHO cells expressing the human D4 receptor[1]. In the PD-168077-treated cell, p-CaMKII exhibits a significantly increased clustering at synaptic sites, as indicated by the enhanced colocalization with PSD-95[2]. |
| In Vivo | PD-168077 (0.2-25.0 mg/kg) dose-dependently induces locomotion, which takes an unusual and characteristic ”shuffling” form with uncoordinated movements together with yawning, and episodes of myoclonic jerking; grooming, and rearing are reduced[1]. |
| References |
| Molecular Formula | C24H26N4O5 |
|---|---|
| Molecular Weight | 450.487 |
| Exact Mass | 450.190308 |
| Storage condition | 2-8℃ |
| N-{[4-(2-Cyanophenyl)-1-piperazinyl]methyl}-3-methylbenzamide (2Z)-2-butenedioate (1:1) |
| N-{[4-(2-Cyanophenyl)piperazin-1-yl]methyl}-3-methylbenzamide (2Z)-but-2-enedioate (1:1) |
| Benzamide, N-[[4-(2-cyanophenyl)-1-piperazinyl]methyl]-3-methyl-, (2Z)-2-butenedioate (1:1) |