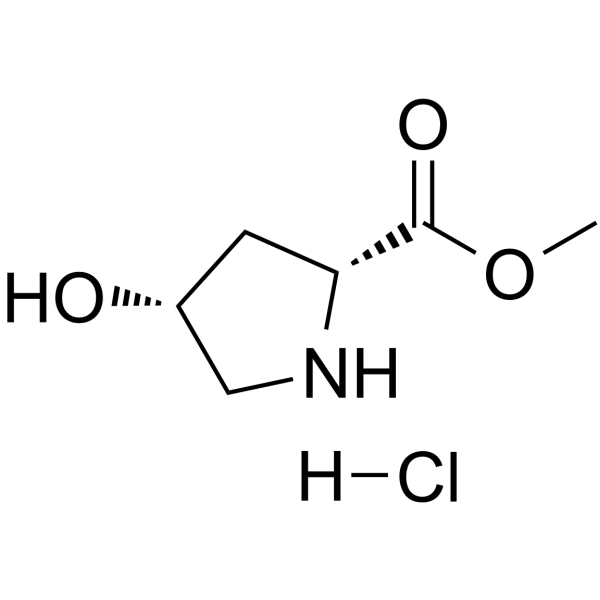
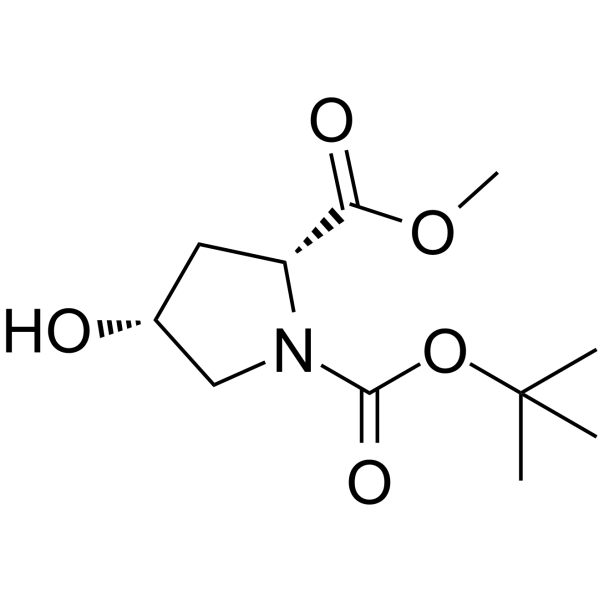
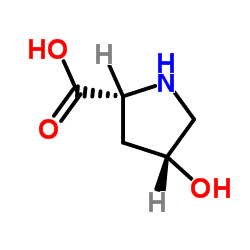
(4S)-1-(tert-Butoxycarbonyl)-4-fluor-D-prolin
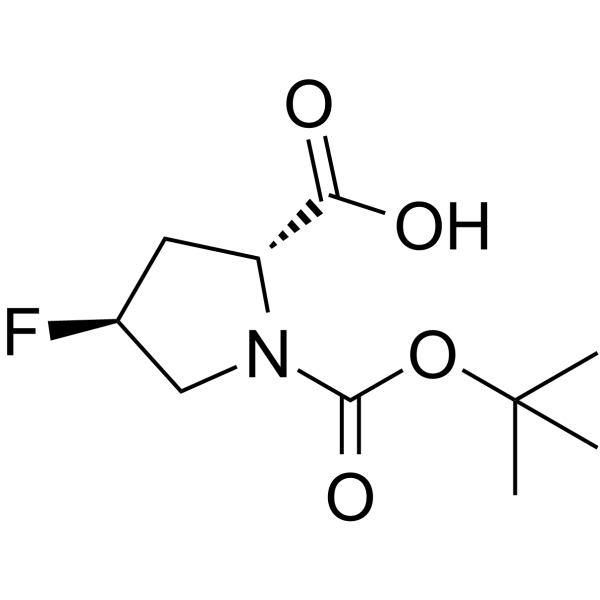
(4S)-1-(tert-Butoxycarbonyl)-4-fluor-D-prolin structure
|
Common Name | (4S)-1-(tert-Butoxycarbonyl)-4-fluor-D-prolin | ||
|---|---|---|---|---|
| CAS Number | 681128-50-7 | Molecular Weight | 233.237 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 346.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H16FNO4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 163.0±27.9 °C | |
Use of (4S)-1-(tert-Butoxycarbonyl)-4-fluor-D-prolin(4S)-1-Boc-4-fluoro-D-proline is a derivative of fluoro-D-proline, can be used for synthesis of compounds[1]. |
| Name | (2R,4S)-4-fluoro-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (4S)-1-Boc-4-fluoro-D-proline is a derivative of fluoro-D-proline, can be used for synthesis of compounds[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Jennifer Elizabeth GOLDEN, et al. Inhibitors of encephalitic alphaviruses. WO2021046315A2. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 346.0±42.0 °C at 760 mmHg |
| Molecular Formula | C10H16FNO4 |
| Molecular Weight | 233.237 |
| Flash Point | 163.0±27.9 °C |
| Exact Mass | 233.106339 |
| PSA | 66.84000 |
| LogP | 0.48 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.488 |
| InChIKey | YGWZXQOYEBWUTH-NKWVEPMBSA-N |
| SMILES | CC(C)(C)OC(=O)N1CC(F)CC1C(=O)O |
|
~% 
(4S)-1-(tert-Bu... CAS#:681128-50-7 |
| Literature: COMENTIS, INC.; BILCER, Geoffrey, M.; LILLY, John, C.; SWANSON, Lisa, M. Patent: WO2011/130383 A1, 2011 ; Location in patent: Page/Page column 86 ; WO 2011/130383 A1 |
|
~% 
(4S)-1-(tert-Bu... CAS#:681128-50-7 |
| Literature: COMENTIS, INC.; BILCER, Geoffrey, M.; LILLY, John, C.; SWANSON, Lisa, M. Patent: WO2011/130383 A1, 2011 ; WO 2011/130383 A1 |
|
~% 
(4S)-1-(tert-Bu... CAS#:681128-50-7 |
| Literature: COMENTIS, INC.; BILCER, Geoffrey, M.; LILLY, John, C.; SWANSON, Lisa, M. Patent: WO2011/130383 A1, 2011 ; WO 2011/130383 A1 |
|
~% 
(4S)-1-(tert-Bu... CAS#:681128-50-7 |
| Literature: COMENTIS, INC.; BILCER, Geoffrey, M.; LILLY, John, C.; SWANSON, Lisa, M. Patent: WO2011/130383 A1, 2011 ; WO 2011/130383 A1 |
| (4S)-1-(tert-butoxycarbonyl)-4-fluoro-D-proline |
| (4S)-4-Fluoro-1-{[(2-methyl-2-propanyl)oxy]carbonyl}-D-proline |
| (4S)-1-(tert-Butoxycarbonyl)-4-fluor-D-prolin |
| 1,2-Pyrrolidinedicarboxylic acid, 4-fluoro-, 1-(1,1-dimethylethyl) ester, (2R,4S)- |