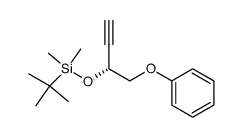
Enprostil
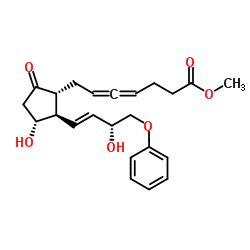
Enprostil structure
|
Common Name | Enprostil | ||
|---|---|---|---|---|
| CAS Number | 73121-56-9 | Molecular Weight | 400.46 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 552.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C23H28O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 185.7±23.6 °C | |
Use of EnprostilEnprostil (RS 84135) is a prostaglandin E2 derivative. Enprostil can inhibit amogastrin-stimulated gastric acid secretion, as well as reducing the secretion of pepsin. Enprostil can also serve as an antiulcer agent, used for research of duodenal or gastric ulcers[1][2]. |
| Name | methyl 7-[(1R,2R,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4-phenoxybut-1-enyl]-5-oxocyclopentyl]hepta-4,5-dienoate |
|---|---|
| Synonym | More Synonyms |
| Description | Enprostil (RS 84135) is a prostaglandin E2 derivative. Enprostil can inhibit amogastrin-stimulated gastric acid secretion, as well as reducing the secretion of pepsin. Enprostil can also serve as an antiulcer agent, used for research of duodenal or gastric ulcers[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.9±50.0 °C at 760 mmHg |
| Molecular Formula | C23H28O6 |
| Molecular Weight | 400.46 |
| Flash Point | 185.7±23.6 °C |
| Exact Mass | 400.188599 |
| PSA | 93.06000 |
| LogP | 1.45 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.586 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~67% 
Enprostil CAS#:73121-56-9 |
| Literature: Gooding, Owen W.; Beard, Colin C.; Cooper, Gary F.; Jackson, David Y. Journal of Organic Chemistry, 1993 , vol. 58, # 14 p. 3681 - 3686 |
|
~% 
Enprostil CAS#:73121-56-9 |
| Literature: Synthesis, , # 8 p. 792 - 794 |
|
~% 
Enprostil CAS#:73121-56-9 |
| Literature: Synthesis, , # 8 p. 792 - 794 |
|
~% 
Enprostil CAS#:73121-56-9 |
| Literature: Synthesis, , # 8 p. 792 - 794 |
|
~% 
Enprostil CAS#:73121-56-9 |
| Literature: Journal of the Chemical Society, Chemical Communications, , # 10 p. 1251 - 1252 |
|
~% 
Enprostil CAS#:73121-56-9 |
| Literature: Journal of the Chemical Society, Chemical Communications, , # 10 p. 1251 - 1252 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| Enprostil (JAN/USAN/INN) |
| 4,5-Heptadienoic acid, 7-[(1R,2R,3R)-3-hydroxy-2-[(1E,3R)-3-hydroxy-4-phenoxy-1-buten-1-yl]-5-oxocyclopentyl]-, methyl ester |
| Syngard |
| Enprostil |
| (dl)-9-Keto-11a,15a-dihydroxy-16-phenoxy-17,18,19,20-tetranorprosta-4,5,13-trans-trienoic Acid Methyl Ester |
| Camleed (TN) |
| methyl (4,5,6S)-7-<(1R,2R,3R)-3-hydroxy-2-<(E)-(3R)-3-hydroxy-4-phenoxy-1-butenyl>-5-oxocyclopentyl>-4,5-heptadienoate |
| Gardrine |
| Methyl 7-{(1R,2R,3R)-3-hydroxy-2-[(1E,3R)-3-hydroxy-4-phenoxy-1-buten-1-yl]-5-oxocyclopentyl}-4,5-heptadienoate |
| [1a,2b(1E,3R*),3a]-7-[3-Hydroxy-2-(3-hydroxy-4-phenoxy-1-butenyl)-5-oxocyclopentyl]-4,5-heptadienoic Acid Methyl Ester |
| methyl (4,5,6R)-7-<(1R,2R,3R)-3-hydroxy-2-<(E)-(3R)-3-hydroxy-4-phenoxy-1-butenyl>-5-oxocyclopentyl>-4,5-heptadienoate |
| Camleed |
| Gardrin |
| Methyl 7-{(1R,2R,3R)-3-hydroxy-2-[(1E,3R)-3-hydroxy-4-phenoxybut-1-en-1-yl]-5-oxocyclopentyl}hepta-4,5-dienoate |
![(3aR,4R,5R,6aS)-4-((3R,E)-4-phenoxy-3-((tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-5-((tetrahydro-2H-pyran-2-yl)oxy)hexahydro-2H-cyclopenta[b]furan-2-ol structure](https://image.chemsrc.com/caspic/169/286840-19-5.png)
![(3aR,4R,5R,6aS)-Hexahydro-4-[(E)-(3R)-4-phenoxy-3-[(tetrahydro-2H-pyran-2-yl)oxy]-1-butenyl]-5-[(tetrahydro-2H-pyran-2-yl)oxy]-2H-cyclopenta[b]furan-2-one structure](https://image.chemsrc.com/caspic/353/54347-99-8.png)
![1-[5α-hydroxy-2β-[(E)-4-phenoxy-3α-tetrahydropyran-2-yloxy-1-butenyl]-3α-tetrahydropyran-2-yloxycyclopent-1α-yl]but-3-yn-2-ol structure](https://image.chemsrc.com/caspic/314/286840-20-8.png)