Antibiotic 1166C
Modify Date: 2025-08-25 19:17:26
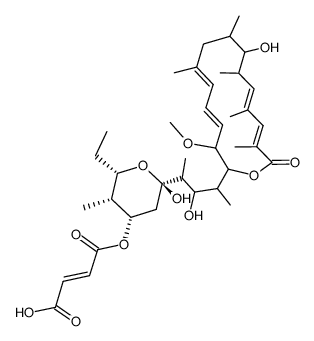
Antibiotic 1166C structure
|
Common Name | Antibiotic 1166C | ||
|---|---|---|---|---|
| CAS Number | 83329-73-1 | Molecular Weight | 690.86100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C38H58O11 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Antibiotic 1166CHygrolidin is a 16-membered macrolide antibiotic produced by Streptomyces hygroscopicus D-1166. Hygrolidin has anti-fungus activity against Valsa ceratosperma. Hygrolidin induces p21 expression and abrogates cell cycle progression at G1 and S phases. Hygrolidin has antitumor activity[1][2][3]. |
| Name | hygrolidin |
|---|
| Description | Hygrolidin is a 16-membered macrolide antibiotic produced by Streptomyces hygroscopicus D-1166. Hygrolidin has anti-fungus activity against Valsa ceratosperma. Hygrolidin induces p21 expression and abrogates cell cycle progression at G1 and S phases. Hygrolidin has antitumor activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Hygrolidin (0.1, 1 μg/ml; for 24 h) increases both G1 and S phase populations and decreases M phase population[1]. Hygrolidin (0.1, 1, 10 μg/ml; for 24 h) decreases the amounts of cdk4, cyclin D, cyclin B and increases the amounts of cyclin E and p21. Hygrolidin-induced p21 preferentially associates with cyclin A-cdk2 complex and inhibits it[1]. Hygrolidin (0.1, 1 μg/ml; for 24 h) selectively induces p21 in DLD-1 cells at mRNA level, but not in WI-38 fibroblasts[1]. Hygrolidin, for 3 days, inhibits growth of various cell lines including DLD-1 colon cancer, LNCaP prostate cancer, K562 leukemia cells, LNCaP prostate cancer, EL-4 lymphoma (IC50s = 1.0-33 ng/ml, respectively)[1]. Hygrolidin shows growth inhibition on Trypanosoma cruzi (IC50=1.1 nM), Trypanosoma brucei brucei (IC50=77 nM), and Leishmania donovani (IC50=72.5 nM)[3]. Cell Cycle Analysis[1] Cell Line: DLD-1 cells Concentration: 0.1, 1 μg/ml Incubation Time: For 24 hours Result: Increased both G1 and S phase populations and decreased M phase population. Western Blot Analysis[1] Cell Line: DLD-1 cells Concentration: 0.1, 1, 10 μg/ml Incubation Time: For 24 hours Result: Decreased the amounts of cdk4, cyclin D, cyclin B and increased the amounts of cyclin E and p21. RT-PCR[1] Cell Line: DLD-1 or WI-38 cells Concentration: 0.1, 1 μg/ml Incubation Time: For 24 hours Result: Selectively induced p21 in DLD-1 cells at mRNA level, but not in WI-38 fibroblasts. |
| References |
| Molecular Formula | C38H58O11 |
|---|---|
| Molecular Weight | 690.86100 |
| Exact Mass | 690.39800 |
| PSA | 169.05000 |
| LogP | 5.05440 |
| InChIKey | UGIOXKVQFJIRMZ-HRNPMOGOSA-N |
| SMILES | CCC1OC(O)(C(C)C(O)C(C)C2OC(=O)C(C)=CC(C)=CC(C)C(O)C(C)CC(C)=CC=CC2OC)CC(OC(=O)C=CC(=O)O)C1C |