Valnivudine
Modify Date: 2025-11-28 15:03:15
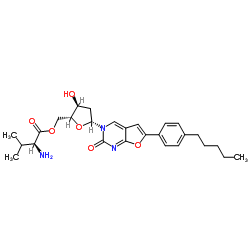
Valnivudine structure
|
Common Name | Valnivudine | ||
|---|---|---|---|---|
| CAS Number | 956483-02-6 | Molecular Weight | 497.583 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 667.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C27H35N3O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 357.4±34.3 °C | |
Use of ValnivudineValnivudine (FV-100 free base), a prodrug of CF-1743, is an orally active anti-herpes zoster (HZ) nucleoside analogue. CF-1743, a bicyclic nucleoside analog (BCNA), has highly specific antiviral activity against varicella-zoster virus (VZV). Valnivudine is rapidly and extensively converted to CF-1743 in vivo[1][2]. |
| Name | [(2R,3S,5R)-3-hydroxy-5-[2-oxo-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-3-yl]oxolan-2-yl]methyl (2S)-2-amino-3-methylbutanoate |
|---|---|
| Synonym | More Synonyms |
| Description | Valnivudine (FV-100 free base), a prodrug of CF-1743, is an orally active anti-herpes zoster (HZ) nucleoside analogue. CF-1743, a bicyclic nucleoside analog (BCNA), has highly specific antiviral activity against varicella-zoster virus (VZV). Valnivudine is rapidly and extensively converted to CF-1743 in vivo[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | In vitro cytotoxicity studies in normal human primary hepatocytes, keratinocytes, and rapidly dividing HepG2 cells, Valnivudine (FV-100 free base) demonstrates mean 50% cytotoxic concentration values of >10 μM[2]. CF-1743 (compound 4f) has anti-varicella-zoster virus (VZV) activity in VZV OKA (EC50=0.3 nM), VZV YS (EC50=0.1 nM) in HEL cell[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 667.3±65.0 °C at 760 mmHg |
| Molecular Formula | C27H35N3O6 |
| Molecular Weight | 497.583 |
| Flash Point | 357.4±34.3 °C |
| Exact Mass | 497.252594 |
| PSA | 129.81000 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.624 |
| FV-100 free base |
| {(2R,3S,5R)-3-Hydroxy-5-[2-oxo-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-3(2H)-yl]tetrahydro-2-furanyl}methyl (2S)-2-amino-3-methylbutanoate |
| UNII-0NJ5F6D4U7 |