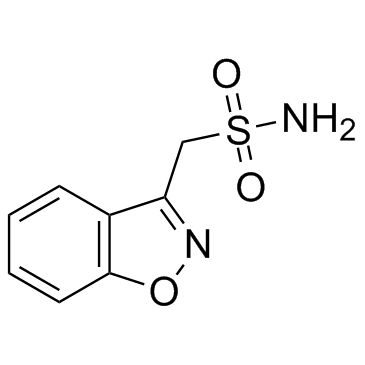
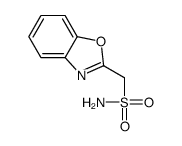
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DE4930000
-
CHEMICAL NAME :
-
1,2-Benzisoxazole-3-methanesulfonamide
-
CAS REGISTRY NUMBER :
-
68291-97-4
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C8-H8-N2-O3-S
-
MOLECULAR WEIGHT :
-
212.24
-
WISWESSER LINE NOTATION :
-
T56 BONJ D1SZW
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1992 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Behavioral - ataxia
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4337,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
733 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,477,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
925 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Behavioral - ataxia
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4337,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
672 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Behavioral - ataxia
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4337,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1829 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #4172896
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
699 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,477,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1009 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Behavioral - ataxia
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4337,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
816 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,600,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - ataxia Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4337,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - smooth muscle relaxant (mechanism undefined, spasmolytic) Behavioral - ataxia Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4337,1987 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5200 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - other changes Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4347,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
23400 mg/kg/39W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4361,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
27375 mg/kg/52W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Blood - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 11,333,1988 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
13 mg/kg
-
SEX/DURATION :
-
male 64 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4387,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5160 mg/kg
-
SEX/DURATION :
-
male 9 week(s) pre-mating female 15 day(s) pre-mating - 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4387,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
220 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Effects on Newborn - behavioral Reproductive - Effects on Newborn - delayed effects
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4399,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
660 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - other postnatal measures or effects
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4399,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2500 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4435,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4435,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1320 mg/kg
-
SEX/DURATION :
-
female 14-35 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4435,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1320 mg/kg
-
SEX/DURATION :
-
female 14-35 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 15,4435,1987
|