|
~0% |
|
~66% |
|
~% |
|
~% |
|
~62% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~60% |
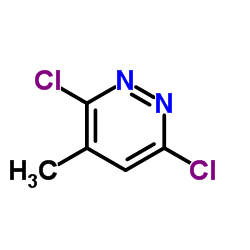
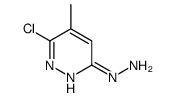
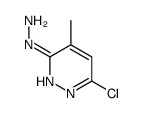
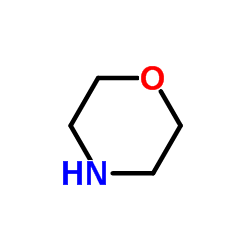
![6-氯-8-甲基-[1,2,4]三唑并[4,3-b]哒嗪结构式](https://image.chemsrc.com/caspic/354/58826-40-7.png)
![4-(8-methyl-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)morpholine结构式](https://image.chemsrc.com/caspic/336/56383-06-3.png)

![6-氯-7-甲基-[1,2,4]三唑并[4,3-b]哒嗪结构式](https://image.chemsrc.com/caspic/209/58826-39-4.png)
![6-chloro-7-methyltetrazolo[1,5-b]pyridazine结构式](https://image.chemsrc.com/caspic/255/28691-22-7.png)
![6-chloro-8-methyltetrazolo[1,5-b]pyridazine结构式](https://image.chemsrc.com/caspic/340/37068-70-5.png)