| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甲酸
CAS:64-18-6 |
|
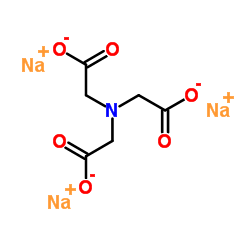 |
次氮基三乙酸钠盐
CAS:5064-31-3 |
|
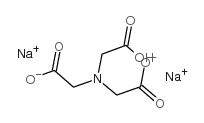 |
次氮基三乙酸二钠盐
CAS:15467-20-6 |
|
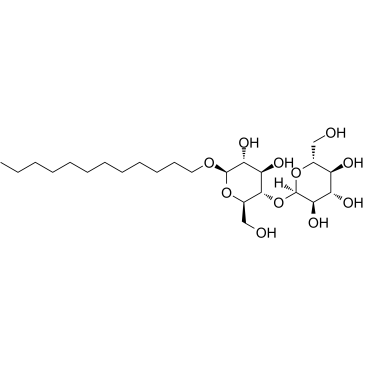 |
十二烷基-beta-D-麦芽糖苷
CAS:69227-93-6 |