| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
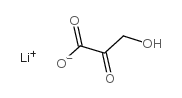 |
Β-羟基丙酮酸锂 水合物
CAS:3369-79-7 |
|
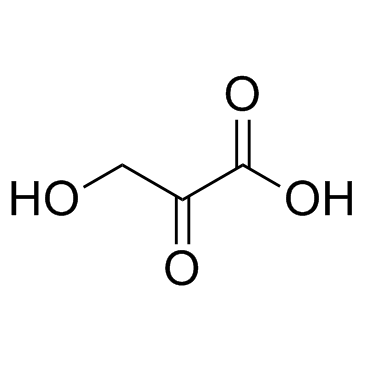 |
羟基丙酮酸
CAS:1113-60-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
Β-羟基丙酮酸锂 水合物
CAS:3369-79-7 |
|
 |
羟基丙酮酸
CAS:1113-60-6 |