Methods in Molecular Biology
2011-01-01
Preparation of peptide and other biomolecular conjugates through chemoselective ligations.
Mathieu Galibert, Olivier Renaudet, Didier Boturyn, Pascal Dumy
文献索引:Methods Mol. Biol. 751 , 67-79, (2011)
全文:HTML全文
摘要
The synthesis of molecular conjugates through chemoselective ligations represents a very convenient strategy to prepare complex macromolecules with diverse functional elements. Herein, we describe chemical methods based on the preparation of chemoselectively addressable peptides allowing successive oxime ligations and/or alkyne-azide cycloaddition ("click") reactions of various biomolecules. This modular synthetic approach can be applied to a broad range of purposes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
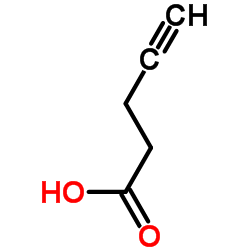 |
4-戊炔酸
CAS:6089-09-4 |
C5H6O2 |
相关文献:
更多...
|
Synthesis, antiproliferative and antifungal activities of 1,...
2015-01-01 [Molecules 20 , 8666-86, (2015)] |
|
In situ nanofabrication of hybrid PEG-dendritic-inorganic na...
2015-03-07 [Nanoscale 7(9) , 3933-40, (2015)] |
|
Surface functionalization of exosomes using click chemistry.
2014-10-15 [Bioconjug. Chem. 25(10) , 1777-84, (2014)] |
|
Parahydrogen-induced polarization of carboxylic acids: a pil...
2014-07-01 [NMR Biomed. 27(7) , 810-6, (2014)] |
|
5-[4-(3,3-Dimethylbutoxycarbonyl)phenyl]-4-pentynoic acid an...
2000-03-01 [Bioorg. Med. Chem. 8(3) , 665-74, (2000)] |