| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
L-半胱氨酸
CAS:52-90-4 |
|
 |
法莫替丁
CAS:76824-35-6 |
|
 |
硫酚
CAS:108-98-5 |
|
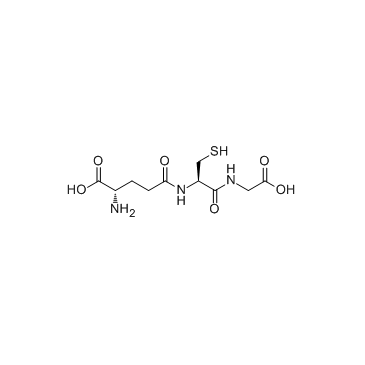 |
谷胱甘肽/5-L-谷氨酰-L-半胱氨酰甘氨酸
CAS:70-18-8 |