| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-cysteine
CAS:52-90-4 |
|
 |
Famotidine
CAS:76824-35-6 |
|
 |
Thiophenol
CAS:108-98-5 |
|
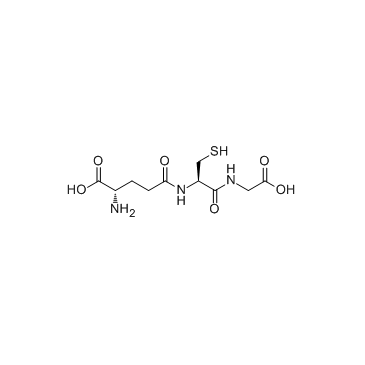 |
Glutathione
CAS:70-18-8 |