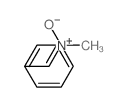
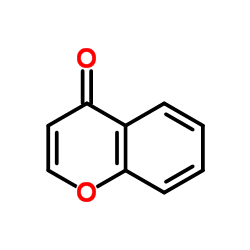
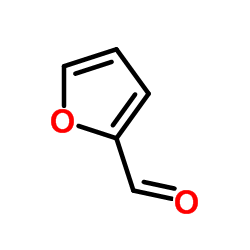
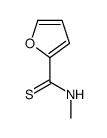
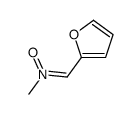
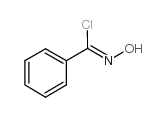
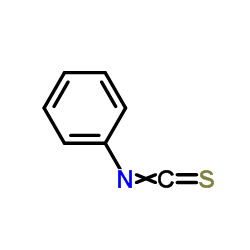
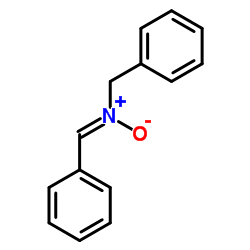
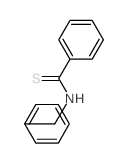
|
~% |
|
~51% |
|
~% |
|
~45% |
|
~78% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~39% |
|
~0% |
|
~45% |
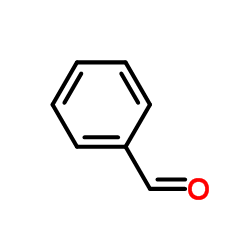
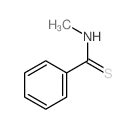
![3-bromo-N-[2-(4-fluorophenyl)benzotriazol-5-yl]-4-methoxybenzamide结构式](https://image.chemsrc.com/caspic/285/6005-15-8.png)