Methemoglobin formation in human erythrocytes by nitroaromatic explosives.
A Maroziene, R Kliukiene, J Sarlauskas, N Cenas
文献索引:Z. Naturforsch., C, J. Biosci. 56(11-12) , 1157-63, (2001)
全文:HTML全文
摘要
We have examined the structure-activity relationships in methemoglobin (MetHb) formation by high explosives 2,4,6-trinitrotoluene (TNT), 2,4,6-trinitrophenyl-N-nitramine (tetryl) and 2,4,6-trinitrophenyl-N-nitraminoethylnitrate (pentryl), and a number of model nitrobenzenes. In lysed human erythrocytes the rate constants of oxyhemoglobin (OxyHb) oxidation increased with an increase in single-electron reduction potential (E(1)7) or with a decrease of the enthalpies of single-electron reduction of nitroaromatics. Tetryl and pentryl oxidized OxyHb almost 3 times faster than TNT. Although the initial rates of MetHb formation in intact erythrocytes by tetryl, pentryl, and TNT matched their order of reactivity in the oxidation of OxyHb in lysed erythrocytes, TNT was a more efficient MetHb forming agent than tetryl and pentryl during a 24-h incubation. The decreased efficiency of tetryl and pentryl was attributed to their reaction with intraerythrocyte reduced glutathione (GSH) producing 2,4,6-trinitrophenyl-Sglutathione, which acted as a less efficient OxyHb oxidizing agent.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
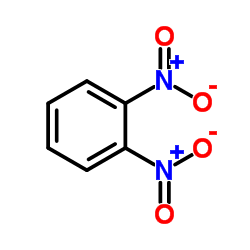 |
1,2-二硝基苯
CAS:528-29-0 |
C6H4N2O4 |
|
Toxicological aspects of photocatalytic degradation of selec...
2015-08-01 [Aquat. Toxicol. 165 , 144-53, (2015)] |
|
Functional characterization of glutathione S-transferases as...
2015-06-01 [Pestic. Biochem. Physiol. 121 , 53-60, (2015)] |
|
Ameliorative action of curcumin in cisplatin-mediated hepato...
2014-08-01 [Arch. Med. Res. 45(6) , 462-8, (2014)] |
|
Reconstitution of TCR alpha-chain expression in deletion mut...
1995-02-15 [J. Immunol. 154(4) , 1551-9, (1995)] |
|
Mutagenic reactivities of 3,4-dinitrobiphenyl derivatives.
1987-06-01 [Mutat. Res. 191(2) , 73-8, (1987)] |