Chirality
2000-01-01
Unprecedented crystallization and X-ray crystal structure of racemic N(alpha)-(t-butyloxycarbonyl)-L-L-phenylalanine N-methoxy-N-methylamide.
X Zheng, I O Donkor, D D Miller, C R Ross
文献索引:Chirality 12(1) , 2-6, (2000)
全文:HTML全文
摘要
Weinreb amide 3 was synthesized using isobutyl chloroformate, carbonyldiimidazole, or ethylcarbodiimide as the coupling agent in the reaction of Boc-phenylalanine with O,N-dimethylhydroxylamine hydrochloride. An optically active oil was isolated along with an optically inactive solid irrespective of the type of coupling agent used. Single crystal X-ray analysis of the solid revealed that it is a racemate. The molecular packing of the crystals reflect the stability of the racemate as opposed to an enantiomerically pure solid.Copyright 2000 Wiley-Liss, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
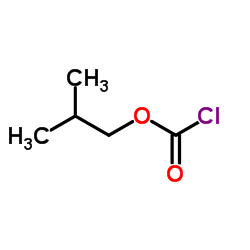 |
氯甲酸异丁酯
CAS:543-27-1 |
C5H9ClO2 |
相关文献:
更多...
|
A new analytical method to determine non-steroidal anti-infl...
2014-11-01 [Talanta 129 , 552-9, (2014)] |
|
Homogeneous detection of caspase-3 using intrinsic fluoresce...
2015-05-15 [Biosens. Bioelectron. 67 , 413-8, (2015)] |
|
Design, synthesis and biological evaluation of novel pyrenyl...
2015-01-07 [Eur. J. Med. Chem. 89 , 851-62, (2014)] |
|
Preparation and Characterization of a Monoclonal Antibody Ag...
2015-08-01 [Monoclon. Antib. Immunodiagn. Immunother. 34 , 270-4, (2015)] |
|
Preparation of intravenous injection nanoformulation of VESy...
2015-11-30 [Int. J. Pharm. 495 , 792-7, (2015)] |