| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
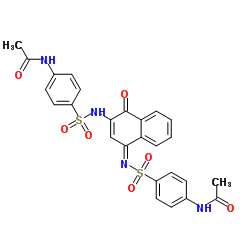 |
酰基辅酶A合成酶
CAS:9013-18-7 |
|
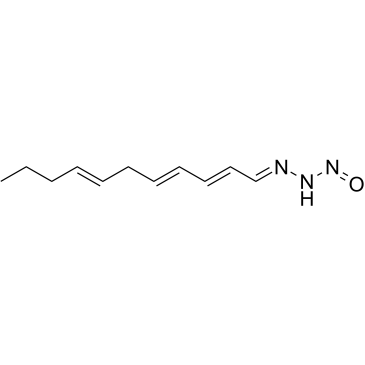 |
三氮菌素 C
CAS:76896-80-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
酰基辅酶A合成酶
CAS:9013-18-7 |
|
 |
三氮菌素 C
CAS:76896-80-5 |