3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles, a novel series of platelet activating factor antagonists.
G S Sheppard, D Pireh, G M Carrera, M G Bures, H R Heyman, D H Steinman, S K Davidsen, J G Phillips, D E Guinn, P D May
文献索引:J. Med. Chem. 37 , 2011, (1994)
全文:HTML全文
摘要
(2RS,4R)-3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles represent a new class of potent, orally active antagonists of platelet activating factor (PAF). The compounds were prepared by acylation of the magnesium or zinc salts of substituted indoles with (2RS,4R)-2-(3-pyridinyl)-3-(tert-butoxycarbonyl)thiazolidin-4-oyl chloride. The 3-acylindole moiety functions as a hydrolytically stabilized and conformationally restricted anilide replacement, which imparts a considerable boost in potency to the series. Structure-activity relationships observed for substitution on the indole ring system are discussed. Members of the series compare favorably with other reported PAF antagonists.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
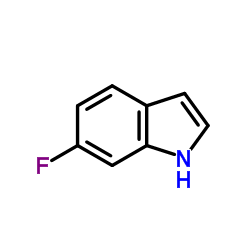 |
6-氟吲哚
CAS:399-51-9 |
C8H6FN |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)e...
2011-08-11 [J. Med. Chem. 54 , 5320, (2011)] |
|
Ionization potentials of fluoroindoles and the origin of non...
2005-03-23 [J. Am. Chem. Soc. 127(11) , 4104-13, (2005)] |
|
Discovery of novel N-β-D-xylosylindole derivatives as sodium...
2011-01-13 [J. Med. Chem. 54 , 166, (2011)] |
|
Synthesis and evaluation of 1-(1H-indol-3-yl)ethanamine deri...
2011-05-15 [Bioorg. Med. Chem. 19 , 3204, (2011)] |
|
New indole derivatives as potent and selective serotonin upt...
1993-04-30 [J. Med. Chem. 36 , 1194, (1993)] |