Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process.
Min-Soo Kim, Shun-Ji Jin, Jeong-Soo Kim, Hee Jun Park, Ha-Seung Song, Reinhard H H Neubert, Sung-Joo Hwang
文献索引:Eur. J. Pharm. Biopharm. 69(2) , 454-65, (2008)
全文:HTML全文
摘要
In this work, amorphous atorvastatin calcium nanoparticles were successfully prepared using the supercritical antisolvent (SAS) process. The effect of process variables on particle size and distribution of atorvastatin calcium during particle formation was investigated. Solid state characterization, solubility, intrinsic dissolution, powder dissolution studies and pharmacokinetic study in rats were performed. Spherical particles with mean particle size ranging between 152 and 863 nm were obtained by varying process parameters such as precipitation vessel pressure and temperature, drug solution concentration and feed rate ratio of CO2/drug solution. XRD, TGA, FT-IR, FT-Raman, NMR and HPLC analysis indicated that atorvastatin calcium existed as anhydrous amorphous form and no degradation occurred after SAS process. When compared with crystalline form (unprocessed drug), amorphous atorvastatin calcium nanoparticles were of better performance in solubility and intrinsic dissolution rate, resulting in higher solubility and faster dissolution rate. In addition, intrinsic dissolution rate showed a good correlation with the solubility. The dissolution rates of amorphous atorvastatin calcium nanoparticles were highly increased in comparison with unprocessed drug by the enhancement of intrinsic dissolution rate and the reduction of particle size resulting in an increased specific surface area. The absorption of atorvastatin calcium after oral administration of amorphous atorvastatin calcium nanoparticles to rats was markedly increased.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
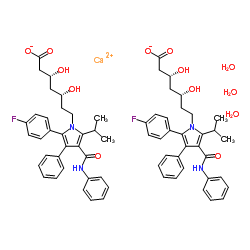 |
阿托伐他汀钙三水合物
CAS:344423-98-9 |
C66H74CaF2N4O13 |
|
Statins demonstrate a broad anti-cytomegalovirus activity in...
2015-01-01 [J. Med. Virol. 87(1) , 141-53, (2014)] |
|
Dehydroepiandrosterone sulfate, a useful endogenous probe fo...
2015-04-01 [Drug Metab. Pharmacokinet. 30(2) , 198-204, (2015)] |
|
Legumain expression, activity and secretion are increased du...
2015-01-01 [Biol. Chem. 396(1) , 71-80, (2014)] |
|
Macitentan does not interfere with hepatic bile salt transpo...
2014-07-01 [J. Pharmacol. Exp. Ther. 350(1) , 130-43, (2014)] |
|
Synergistic effect of hydrotrope and surfactant on solubilit...
2014-01-01 [Indian J. Pharm. Sci. 76(6) , 483-94, (2015)] |