| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
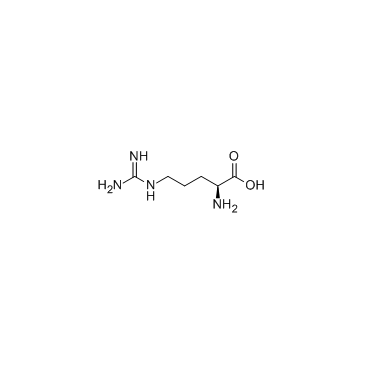 |
L-精氨酸
CAS:74-79-3 |
|
 |
肌黄酶 来源于梭状芽孢杆菌
CAS:9001-18-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
L-精氨酸
CAS:74-79-3 |
|
 |
肌黄酶 来源于梭状芽孢杆菌
CAS:9001-18-7 |