Organic Letters
2011-09-02
Stereoselective synthesis of substituted 1,3-oxazolidines via Pd-catalyzed carboamination reactions of O-vinyl-1,2-amino alcohols.
Amanda F Ward, John P Wolfe
文献索引:Org. Lett. 17th ed., 13 , 4728-4731, (2011)
全文:HTML全文
摘要
The stereoselective synthesis of 2,4- and 2,5-disubstituted 1,3-oxazolidines is accomplished via Pd-catalyzed carboamination of O-vinyl-1,2-amino alcohol derivatives. The transformations generate cis-disubstituted products with good to excellent diastereoselectivity, and enantiomerically enriched substrates are converted without loss of optical purity. In addition to yielding synthetically useful products that are difficult to generate with existing methods, these transformations illustrate that electron-rich enol ethers are viable substrates for alkene carboamination processes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
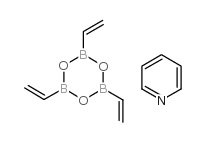 |
乙烯硼酐吡啶络合物
CAS:442850-89-7 |
C11H14B3NO3 |
相关文献:
更多...
|
Transition metal-catalyzed synthesis and reactivity of N-alk...
2005-03-17 [Org. Lett. 6th ed., 7 , 1161-1164, (2005)] |
|
Stereoselective isomerisation of N-allyl aziridines into geo...
2008-01-01 [Chemistry 3rd ed., 14 , 886-894, (2008)] |
|
The alkyl-connected 2-amino-6-vinylpurine (AVP) crosslinking...
2010-01-01 [Bioorg. Med. Chem. 8th ed., 18 , 2894-2901, (2010)] |
|
Kinetic resolution of axially chiral 2,2'-dihydroxy-1,1'-bia...
2005-08-03 [J. Am. Chem. Soc. 30th ed., 127 , 10474-10475, (2005)] |
|
Efforts toward distorted spiropentanes.
2010-11-05 [J. Org. Chem. 21th ed., 75 , 7494-7497, (2010)] |