Identification of a reactive lysyl residue (Lys103) of recombinant human interleukin-1 beta. Mechanism of its reactivity and implication of its functional role in receptor binding.
J Y Chang, P K Ngai, J P Priestle, U Joss, K D Vosbeck, J van Oostrum
文献索引:Biochemistry 31(11) , 2874-8, (1992)
全文:HTML全文
摘要
Recombinant human interleukin-1 beta (h-IL-1 beta) was chemically modified with 4-(N,N-dimethylamino)-4'-isothiocyanatoazobenzene-2'-sulfonic acid (S-DABITC), a water-soluble color reagent specific for lysine labeling. Modified h-IL-1 beta was digested by lysyl endopeptidase. Peptides containing labeled lysines were detected at the visible wavelength (436 nm) and isolated by HPLC. The modification sites were eventually determined by sequence analysis. The results revealed that Lys103, Lys92, Lys93, and Lys94 of h-IL-1 beta reacted selectively with S-DABITC. A 1-h incubation with 1 mM S-DABITC at room temperature resulted in a quantitative modification of Lys103, 22% of Lys92, 27% of Lys93, and 18% of Lys94, respectively. This modification was accompanied by a 20-fold decrease of the protein's ability to bind to the receptor. Furthermore, a mutant of h-IL-1 beta (M9, Glu105 substituted by Lys) exhibits markedly impaired receptor binding, and the S-DABITC reactivity of its Lys103 was found to be reduced by 90%. These findings suggest that Lys103 of h-IL-1 beta might play an important role in the h-IL-1 beta/receptor interaction.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
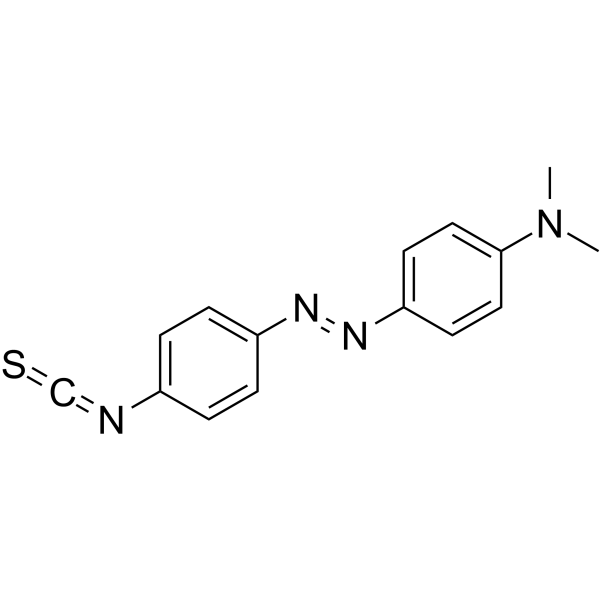 |
4-二甲氨基偶氮苯-4'-异硫氰酸酯
CAS:7612-98-8 |
C15H14N4S |
|
A new isotype sequence (V kappa 27) of the variable region o...
1983-04-01 [Biochem. J. 211(1) , 173-80, (1983)] |
|
Peptide maps at picomolar levels obtained by reversed-phase ...
[J. Chromatogr. A. 548(1-2) , 303-10, (1991)] |
|
Evidence for the formation of a novel glutathione conjugate ...
1984-01-01 [Drug Metab. Dispos. 12(4) , 523-4, (1984)] |
|
Novel histamine measurement by HPLC analysis used to assay h...
1998-08-01 [Biosci. Biotechnol. Biochem. 62(8) , 1488-91, (1998)] |
|
Improvements in the application of the 4,4-N,N-dimethylamino...
1989-08-01 [Anal. Biochem. 180(2) , 374-9, (1989)] |