Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area.
Sung Hwa Shin, Eun Jeoung Lee, Sunghee Hyun, Jaesun Chun, Yangmi Kim, Sang Sun Kang
文献索引:Cell. Signal. 24(3) , 641-51, (2012)
全文:HTML全文
摘要
Previously, we demonstrated that the transient receptor potential vanilloid 4 (TRPV4) cation channel, a member of the TRP vanilloid subfamily, is one of the serum glucocorticoid-induced protein kinase1 (SGK1) authentic substrate proteins, and that the Ser 824 residue of TRPV4 is phosphorylated by SGK1. In this study, we demonstrated that phosphorylation on the Ser 824 residue of TRPV4 is required for its interaction with F-actin, using TRPV4 mutants (S824D; a phospho-mimicking TRPV4 mutant and S824A; a non-phosphorylatable TRPV4 mutant) and its proper subcellular localization. Additionally, we noted that the phosphorylation of the Ser824 residue promotes its single channel activity, Ca(2+) influx, protein stability, and cell surface area (expansion of plasma membrane).Copyright © 2011 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
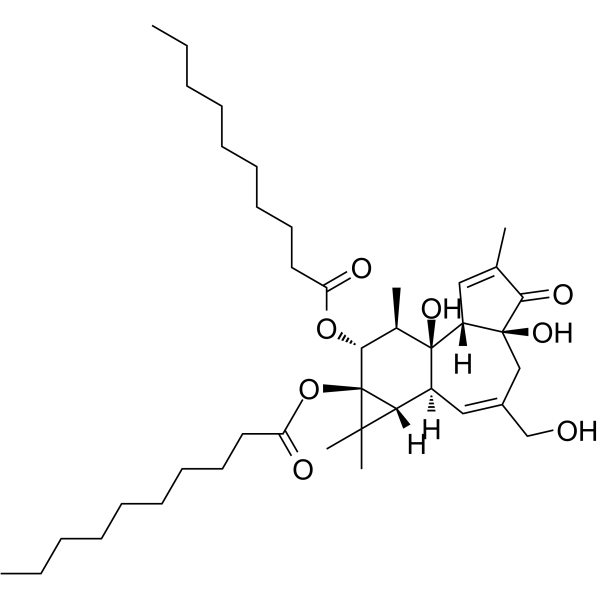 |
4α-佛波醇-12,13-二癸酸酯
CAS:27536-56-7 |
C40H64O8 |
|
Involvement of TRPV1 and TRPV4 channels in migration of rat ...
2012-09-01 [Pflugers Arch. 464(3) , 261-72, (2012)] |
|
Ca2+ entry via alpha1G and TRPV4 channels differentially reg...
2009-10-01 [Am. J. Physiol. Lung Cell. Mol. Physiol. 297(4) , L650-7, (2009)] |
|
Salt intake augments hypotensive effects of transient recept...
2009-02-01 [Hypertension 53(2) , 228-35, (2009)] |
|
Axonal neuropathy-associated TRPV4 regulates neurotrophic fa...
2012-02-17 [J. Biol. Chem. 287(8) , 6014-24, (2012)] |
|
Characterization of the antinociceptive and anti-inflammator...
2007-12-08 [Eur. J. Pharmacol. 576(1-3) , 171-9, (2007)] |