| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
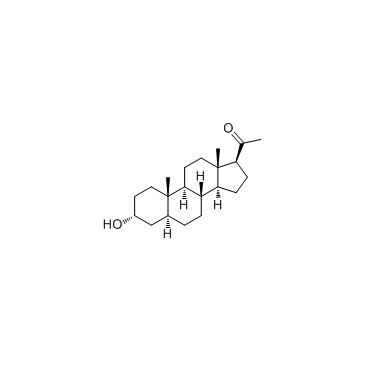 |
5alpha-孕甾-3alpha-醇-20-酮
CAS:516-54-1 |
|
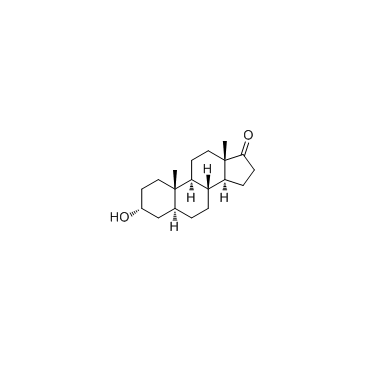 |
雄酮
CAS:53-41-8 |
|
![双环[2.2.2]硫代磷酸叔丁酯 结构式](https://image.chemsrc.com/caspic/095/70636-86-1.png) |
双环[2.2.2]硫代磷酸叔丁酯
CAS:70636-86-1 |
|
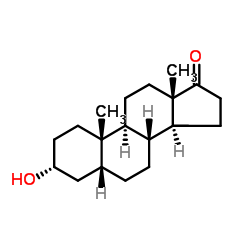 |
3a-羟基-5b-雄甾烷-17-酮
CAS:53-42-9 |