| Structure | Name/CAS No. | Articles |
|---|---|---|
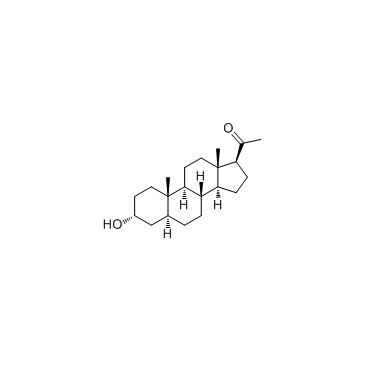 |
5alpha-Pregnan-3alpha-ol-20-one
CAS:516-54-1 |
|
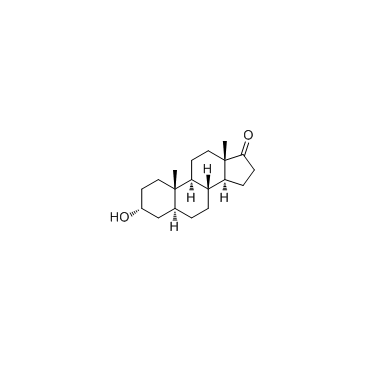 |
Androsterone
CAS:53-41-8 |
|
![TERT-BUTYL-BICYCLO [2.2.2] Structure](https://image.chemsrc.com/caspic/095/70636-86-1.png) |
TERT-BUTYL-BICYCLO [2.2.2]
CAS:70636-86-1 |
|
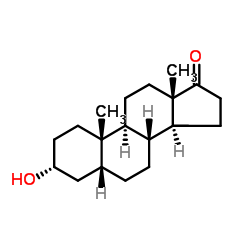 |
Etiocholanolone
CAS:53-42-9 |