Application of Lewis acid catalyzed tropone [6+4] cycloadditions to the synthesis of the core of CP-225,917.
L Isakovic, J A Ashenhurst, J L Gleason
文献索引:Org. Lett. 3(26) , 4189-92, (2001)
全文:HTML全文
摘要
The carbocyclic core of CP-225,917 and CP-263,114 is accessible through the [6+4] cycloaddition of a tropone with a 2-substituted cyclopentadiene. Examination of this reaction has revealed for the first time that this cycloaddition process is catalyzed by Lewis acids, including lanthanide triflates. Cycloadditions of several mono-, di-, and trisubstituted tropones with 2-silyloxycyclopentadienes using ZnCl(2) catalysis are found to proceed in good yield and, in many cases, with excellent diastereoselectivity. Subsequent transformation to the core of the CP-molecules involves a site-selective Baeyer-Villiger oxidation of a tricyclic diketone, followed by a syn-elimination process. [reaction: see text]
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
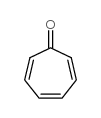 |
环庚三烯酮
CAS:539-80-0 |
C7H6O |
|
Benzo[cd]azulene skeleton: azulene, heptafulvene, and tropon...
2009-12-03 [Org. Lett. 11(23) , 5363-5, (2009)] |
|
Tropylium tetrafluoroborate, a novel substrate for aldehyde ...
1986-10-30 [Biochem. Biophys. Res. Commun. 140(2) , 609-15, (1986)] |
|
Potential of Piperazinylalkylester Prodrugs of 6-Methoxy-2-N...
2015-06-01 [Z. Naturforsch., C, J. Biosci. 59(3-4) , 184-6, (2004)] |
|
Pernambucone, a new tropone derivative from Croton argyroglo...
2009-05-01 [Pharmazie 64(5) , 350-1, (2009)] |
|
Metal-catalyzed [6 + 3] cycloaddition of tropone with azomet...
2014-02-12 [J. Am. Chem. Soc. 136(6) , 2625-9, (2014)] |