The reverse of the 'repair' reaction of thiols: H-abstraction at carbon by thiyl radicals.
M S Akhlaq, H P Schuchmann, C von Sonntag
文献索引:Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 51(1) , 91-102, (1987)
全文:HTML全文
摘要
Thiyl radicals (RS) formed by the reaction of radiolytically generated OH radicals with thiols, e.g. 1,4-dithiothreitol (DTT), react with cis- and trans-2,5-dimethyltetrahydrofuran by abstracting an H atom in the alpha-position to the ether function (k approximately equal to 5 X 10(3) dm3 mol-1 s-1). The so-formed planar ether radical is 'repaired' by the thiol (k = 6 X 10(8) dm3 mol-1 s-1) thereby regenerating a cis- or trans-2,5-dimethyltetrahydrofuran molecule. In this reaction a thiyl radical is reproduced. Thus trans-2,5-Me2THF from cis-2,5-Me2THF and vice versa are formed in a chain reaction: at a dose rate of 2.8 X 10(-3) Gys-1 and a trans-2,5-Me2THF concentration of 1 X 10(-2) mol dm-3 using DTT as the thiol, G(cis-2,5-Me2THF) = 160 has been found. The chain reaction is very sensitive to impurities and also to disulphides such as those radiolytically formed. 2,5-Me2THF can be regarded as a model for the sugar moiety of DNA where the C(4')-radical is known to lead to DNA strand breakage. The possible role of cellular thiols in the repair of the C(4') DNA radical, and also the conceivable role of thiyl radicals inducing DNA strand breakage, are discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
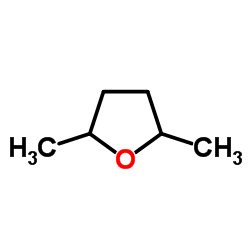 |
2,5-二甲基四氢呋喃
CAS:1003-38-9 |
C6H12O |
|
Activation of transient receptor potential vanilloid 3 chann...
2015-06-01 [Endocrinology 156 , 2074-86, (2015)] |
|
One-step catalytic transformation of carbohydrates and cellu...
2010-05-25 [ChemSusChem 3(5) , 597-603, (2010)] |
|
Mechanistic study of a one-step catalytic conversion of fruc...
2012-09-24 [Chemistry 18(39) , 12363-71, (2012)] |
|
Kinetics and thermochemistry of 2,5-dimethyltetrahydrofuran ...
2012-05-10 [J. Phys. Chem. A 116(18) , 4528-38, (2012)] |
|
Independent generation and reactivity of 2-deoxy-5-methylene...
2000-07-28 [J. Org. Chem. 65(15) , 4648-54, (2000)] |