Independent generation and reactivity of 2-deoxy-5-methyleneuridin-5-yl, a significant reactive intermediate produced from thymidine as a result of oxidative stress.
A S Anderson, J T Hwang, M M Greenberg
文献索引:J. Org. Chem. 65(15) , 4648-54, (2000)
全文:HTML全文
摘要
2'-Deoxy-5-methyleneuridin-5-yl (1) is produced in a variety of DNA damage processes and is believed to result in the formation of lesions that are mutagenic and refractory to enzymatic repair. 2'-Deoxy-5-methyleneuridin-5-yl (1) was independently generated under anaerobic conditions via Norrish Type I photocleavage during Pyrex filtered photolysis of the benzyl ketone 7. The radical (1) exhibits behavior consistent with that of a resonance-stabilized radical. The KIE for hydrogen atom transfer from t-BuSH was found to be 7.3 +/- 1.7. Competition studies between radical recombination and hydrogen atom donors (2,5-dimethyltetrahydrofuran, kTrap = 46.1 +/- 15.4 M(-1) s(-1); propan-2-ol, kTrap = 13.6 +/- 3.5 M(-1) s(-1)) chosen to mimic the carbohydrate components of 2'-deoxyribonucleotides suggest that 2'-deoxy-5-methyleneuridin-5-yl (1) may be able to transfer damage from the nucleobase to the deoxyribose of an adjacent nucleotide in DNA under hypoxic conditions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
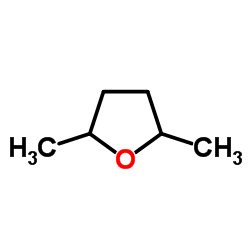 |
2,5-二甲基四氢呋喃
CAS:1003-38-9 |
C6H12O |
|
Activation of transient receptor potential vanilloid 3 chann...
2015-06-01 [Endocrinology 156 , 2074-86, (2015)] |
|
The reverse of the 'repair' reaction of thiols: H-abstractio...
1987-01-01 [Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 51(1) , 91-102, (1987)] |
|
One-step catalytic transformation of carbohydrates and cellu...
2010-05-25 [ChemSusChem 3(5) , 597-603, (2010)] |
|
Mechanistic study of a one-step catalytic conversion of fruc...
2012-09-24 [Chemistry 18(39) , 12363-71, (2012)] |
|
Kinetics and thermochemistry of 2,5-dimethyltetrahydrofuran ...
2012-05-10 [J. Phys. Chem. A 116(18) , 4528-38, (2012)] |