The biosynthesis of the branched-chain sugar d-apiose in plants: functional cloning and characterization of a UDP-d-apiose/UDP-d-xylose synthase from Arabidopsis.
Michael Mølhøj, Rajeev Verma, Wolf-Dieter Reiter
文献索引:Plant J. 35 , 693-703, (2003)
全文:HTML全文
摘要
d-Apiose is a plant-specific branched-chain monosaccharide found in rhamnogalacturonan II (RG-II), apiogalacturonan, and several apioglycosides. Within RG-II, d-apiose serves as the binding site for borate, which leads to the formation of cross-links within the wall. Biochemical studies in duckweed and parsley have established that uridine 5'-diphospho-d-apiose (UDP-d-apiose) is formed from UDP-d-glucuronate by decarboxylation and re-arrangement of the carbon skeleton, leading to ring contraction and branch formation. The enzyme catalyzing this reaction also forms UDP-d-xylose by decarboxylation of UDP-d-glucuronate, and has therefore been named UDP-d-apiose/UDP-d-xylose synthase. Using a bioinformatics approach, we identified a candidate gene (AXS1) for this enzyme in Arabidopsis and functionally expressed its cDNA in Escherichia coli. The recombinant enzyme catalyzed the conversion of UDP-d-glucuronate to a mixture of UDP-d-apiose and UDP-d-xylose with a turnover number of 0.3 min-1. AXS1 required NAD+ for enzymatic activity, and was strongly inhibited by UDP-d-galacturonate. It was highly expressed in all plant organs consistent with a function in synthesizing an essential cell wall precursor. Database searches indicated the presence of closely related sequences in a variety of crop plants. The cloning of the AXS1 gene will help to investigate the biosynthesis of RG-II, and permit insights into the mechanism by which d-apiose and other branched monosaccharides are formed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
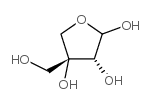 |
3-C-羟基甲基-d-丙三醇-四糖
CAS:639-97-4 |
C5H10O5 |
|
Use of Commercial Dry Yeast Products Rich in Mannoproteins f...
2015-06-17 [J. Agric. Food Chem. 63 , 5670-81, (2015)] |
|
Synthesis of apiose-containing oligosaccharide fragments of ...
2011-10-07 [Org. Biomol. Chem. 9(19) , 6670-84, (2011)] |
|
The acetylation of apiitol in the determination of apiose.
1990-05-15 [Carbohydr. Res. 199(1) , 55-65, (1990)] |
|
cDNA-AFLP analysis on bolting or flowering of flowering Chin...
2012-07-01 [Mol. Biol. Rep. 39(7) , 7525-31, (2012)] |
|
Synthesis and in vitro activity of 4' and 5'-modified analog...
2009-11-01 [Nucleosides Nucleotides Nucleic Acids 28(11) , 1104-16, (2009)] |