3-C-羟基甲基-d-丙三醇-四糖
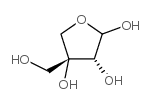
3-C-羟基甲基-d-丙三醇-四糖结构式

|
常用名 | 3-C-羟基甲基-d-丙三醇-四糖 | 英文名 | D-apiose |
|---|---|---|---|---|
| CAS号 | 639-97-4 | 分子量 | 150.13000 | |
| 密度 | 1.711g/cm3 | 沸点 | 364.2ºC at 760 mmHg | |
| 分子式 | C5H10O5 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 174.1ºC |
|
Use of Commercial Dry Yeast Products Rich in Mannoproteins for White and Rosé Sparkling Wine Elaboration.
J. Agric. Food Chem. 63 , 5670-81, (2015) In sparkling wines, mannoproteins released during yeast autolysis largely affect their final quality. This process is very slow and may take several months. The aim of this work was to study the effect of several commercial dry yeast autolysates on the chemic... |
|
|
Synthesis of apiose-containing oligosaccharide fragments of the plant cell wall: fragments of rhamnogalacturonan-II side chains A and B, and apiogalacturonan.
Org. Biomol. Chem. 9(19) , 6670-84, (2011) Fragments of pectic polysaccharides rhamnogalacturonan-II (RG-II) and apiogalacturonan were synthesised using p-tolylthio apiofuranoside derivatives as key building blocks. Apiofuranose thioglycosides can be conveniently prepared by cyclization of the corresp... |
|
|
The acetylation of apiitol in the determination of apiose.
Carbohydr. Res. 199(1) , 55-65, (1990) The complete acetylation of apiitol required 9 h when acetic anhydride at 120 degrees was used and sodium acetate was the catalyst. Both apiitol pentaacetate and apiitol tetraacetate were detected before acetylation was complete. When the reaction was done in... |
|
|
cDNA-AFLP analysis on bolting or flowering of flowering Chinese cabbage and molecular characteristics of BrcuDFR-like/BrcuAXS gene.
Mol. Biol. Rep. 39(7) , 7525-31, (2012) The molecular basis of flower bud differentiation in flowering Chinese cabbage (Brassica rapa L. ssp. Chinensis var. utilis Tsen et Lee) was studied in this work. Samples were taken from two varieties, the early-blooming "Youqin 49" and the late-blooming "You... |
|
|
Synthesis and in vitro activity of 4' and 5'-modified analogues of apiosyl nucleosides as potent anti-HCV agents.
Nucleosides Nucleotides Nucleic Acids 28(11) , 1104-16, (2009) Novel doubly branched apio dideoxynucleosides were synthesized starting from 1,3-dihydroxyacetone using an ozonolysis and Grignard addition as key steps, and evaluated for anti-hepatitis C virus (HCV) activity. The adenine derivative 24 showed significant ant... |
|
|
Simple synthesis and anti-HIV activity of novel 3'-vinyl branched apiosyl pyrimidine nucleosides.
Nucleosides Nucleotides Nucleic Acids 25(8) , 871-8, (2006) Novel vinyl branched apiosyl nucleosides were synthesized in this study. Apiosyl sugar moiety was constructed by sequential ozonolysis and reductions. The bases (uracil and thymine) were efficiently coupled by glycosyl condensation procedure (persilyated base... |
|
|
Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide.
Annu. Rev. Plant Biol. 55 , 109-139, (2004) Rhamnogalacturonan II (RG-II) is a structurally complex pectic polysaccharide that was first identified in 1978 as a quantitatively minor component of suspension-cultured sycamore cell walls. Subsequent studies have shown that RG-II is present in the primary ... |
|
|
Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside.
Curr. Opin. Plant Biol. 7 , 277-284, (2004) Plants possess a sophisticated sugar biosynthetic machinery comprising families of nucleotide sugar interconversion enzymes. Literature published in the past two years has made a major contribution to our knowledge of the enzymes and genes involved in the int... |
|
|
The biosynthesis of the branched-chain sugar d-apiose in plants: functional cloning and characterization of a UDP-d-apiose/UDP-d-xylose synthase from Arabidopsis.
Plant J. 35 , 693-703, (2003) d-Apiose is a plant-specific branched-chain monosaccharide found in rhamnogalacturonan II (RG-II), apiogalacturonan, and several apioglycosides. Within RG-II, d-apiose serves as the binding site for borate, which leads to the formation of cross-links within t... |
|
|
Depletion of UDP-D-apiose/UDP-D-xylose synthases results in rhamnogalacturonan-II deficiency, cell wall thickening, and cell death in higher plants.
J. Biol. Chem. 281 , 13708-13716, (2006) D-apiose serves as the binding site for borate cross-linking of rhamnogalacturonan II (RG-II) in the plant cell wall, and biosynthesis of D-apiose involves UDP-D-apiose/UDP-D-xylose synthase catalyzing the conversion of UDP-D-glucuronate to a mixture of UDP-D... |