Organic Letters
2010-11-19
One-pot enantioselective syntheses of iminosugar derivatives using organocatalytic anti-michael-anti-aza-Henry reactions.
Ritsuo Imashiro, Hisatoshi Uehara, Carlos F Barbas
文献索引:Org. Lett. 12(22) , 5250-3, (2010)
全文:HTML全文
摘要
Organocatalyst-controlled asymmetric anti-Michael reactions of (tert-butyldimethylsilyloxy)acetaldehyde with a range of nitroolefins, followed by an intermolecular aza-Henry reaction with imine, provided iminosugar derivatives with five contiguous stereocenters in very high enantiomeric excess in one pot. The stereochemistry of the aza-Henry reaction was substrate controlled and is explained by a six-membered cyclic transition-state model.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
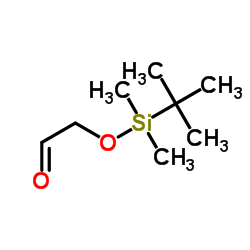 |
叔丁基二甲基硅氧烷基乙醛
CAS:102191-92-4 |
C8H18O2Si |
相关文献:
更多...
|
Enantioselective total synthesis of (-)-dactylolide.
2006-03-16 [Org. Lett. 8(6) , 1117-1120, (2006)] |
|
Organocatalytic asymmetric assembly reactions for the synthe...
2010-11-30 [Proc. Natl. Acad. Sci. U. S. A. 107(48) , 20672-7, (2010)] |
|
Colobert, Francoise; et al.
[European J. Org. Chem. 6 , 1455-1467, (2006)] |
|
Synthesis and Biological Evaluation of the Trifluoromethyl A...
[European J. Org. Chem. 20 , 4731-36, (2006)] |
|
Synthesis of (±)-thioascorbic acid. Stachel H-D, et al.
[Tetrahedron 49(22) , 4871-80, (1993)] |