叔丁基二甲基硅氧烷基乙醛
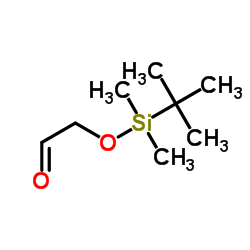
叔丁基二甲基硅氧烷基乙醛结构式

|
常用名 | 叔丁基二甲基硅氧烷基乙醛 | 英文名 | {[Dimethyl(2-methyl-2-propanyl)silyl]oxy}acetaldehyde |
|---|---|---|---|---|
| CAS号 | 102191-92-4 | 分子量 | 174.313 | |
| 密度 | 0.9±0.1 g/cm3 | 沸点 | 161.0±23.0 °C at 760 mmHg | |
| 分子式 | C8H18O2Si | 熔点 | 165-167ºC | |
| MSDS | 中文版 美版 | 闪点 | 42.7±18.2 °C | |
| 符号 |


GHS02, GHS07 |
信号词 | Warning |
|
Enantioselective total synthesis of (-)-dactylolide.
Org. Lett. 8(6) , 1117-1120, (2006) [reaction: see text] The enantioselective total synthesis of (-)-dactylolide is reported. The absolute stereochemistry of the tetrahydropyran was established by catalytic asymmetric Jacobsen hetero-Diels-Alder reaction. The remote C19 stereocenter was introdu... |
|
|
One-pot enantioselective syntheses of iminosugar derivatives using organocatalytic anti-michael-anti-aza-Henry reactions.
Org. Lett. 12(22) , 5250-3, (2010) Organocatalyst-controlled asymmetric anti-Michael reactions of (tert-butyldimethylsilyloxy)acetaldehyde with a range of nitroolefins, followed by an intermolecular aza-Henry reaction with imine, provided iminosugar derivatives with five contiguous stereocente... |
|
|
Organocatalytic asymmetric assembly reactions for the syntheses of carbohydrate derivatives by intermolecular Michael-Henry reactions.
Proc. Natl. Acad. Sci. U. S. A. 107(48) , 20672-7, (2010) Given the significance of carbohydrates in life, medicine, and industry, the development of simple and efficient de novo methods to synthesize carbohydrates are highly desirable. Organocatalytic asymmetric assembly reactions are powerful tools to rapidly cons... |
|
|
Colobert, Francoise; et al.
European J. Org. Chem. 6 , 1455-1467, (2006)
|
|
|
Synthesis and Biological Evaluation of the Trifluoromethyl Analog of (4S)-4, 5-Dihydroxy-2, 3-pentanedione (DPD). Frezza M, et al.
European J. Org. Chem. 20 , 4731-36, (2006)
|
|
|
Synthesis of (±)-thioascorbic acid. Stachel H-D, et al.
Tetrahedron 49(22) , 4871-80, (1993)
|