Use of mass spectrometry to probe the nucleophilicity of cysteinyl residues of prolyl hydroxylase domain 2.
Jasmin Mecinović, Rasheduzzaman Chowdhury, Emily Flashman, Christopher J Schofield
文献索引:Anal. Biochem. 393(2) , 215-21, (2009)
全文:HTML全文
摘要
Prolyl hydroxylase domain 2 (PHD2) plays an important role in hypoxic sensing in humans. Here we report studies on the reactivity of cysteinyl residues of the catalytic domain of PHD2 using an approach in which nondenaturing electrospray ionization-mass spectrometry (ESI-MS) analyses were combined with the use of a thiol library and residue substitution. Among the seven cysteinyl residues of the PHD2 catalytic domain, Cys201 was found to be predominantly modified by thiols or N-ethylmaleimide. Selective modification of Cys201 was further demonstrated with methanethiosulfonate, a spin-labeled probe. The modified PHD2 will be useful in electron paramagnetic resonance studies on PHD2. The results demonstrate the use of a combined library/residue substitution/ESI-MS approach for analyzing residue reactivity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
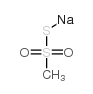 |
硫甲磺酸钠
CAS:1950-85-2 |
CH3NaO2S2 |
|
Large scale production of the active human ASCT2 (SLC1A5) tr...
2013-09-01 [Biochim. Biophys. Acta 1828(9) , 2238-46, (2013)] |
|
About half of the late sodium current in cardiac myocytes fr...
2012-11-01 [J. Mol. Cell. Cardiol. 53(5) , 593-8, (2012)] |
|
Interaction sites of tropomyosin in muscle thin filament as ...
2011-05-18 [Biophys. J. 100(10) , 2432-9, (2011)] |
|
Insights into the mechanism of pore opening of acid-sensing ...
2011-05-06 [J. Biol. Chem. 286(18) , 16297-307, (2011)] |
|
GABA(A) receptor transmembrane amino acids are critical for ...
2014-02-01 [J. Neurochem. 128(3) , 363-75, (2014)] |