Inhibition of endoplasmic reticulum-associated degradation rescues native folding in loss of function protein misfolding diseases.
Fan Wang, Wensi Song, Giovanna Brancati, Laura Segatori
文献索引:J. Biol. Chem. 286(50) , 43454-64, (2011)
全文:HTML全文
摘要
Lysosomal storage disorders are often caused by mutations that destabilize native folding and impair trafficking of secretory proteins. We demonstrate that endoplasmic reticulum (ER)-associated degradation (ERAD) prevents native folding of mutated lysosomal enzymes in patient-derived fibroblasts from two clinically distinct lysosomal storage disorders, namely Gaucher and Tay-Sachs disease. Prolonging ER retention via ERAD inhibition enhanced folding, trafficking, and activity of these unstable enzyme variants. Furthermore, combining ERAD inhibition with enhancement of the cellular folding capacity via proteostasis modulation resulted in synergistic rescue of mutated enzymes. ERAD inhibition was achieved by cell treatment with small molecules that interfere with recognition (kifunensine) or retrotranslocation (eeyarestatin I) of misfolded substrates. These different mechanisms of ERAD inhibition were shown to enhance ER retention of mutated proteins but were associated with dramatically different levels of ER stress, unfolded protein response activation, and unfolded protein response-induced apoptosis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
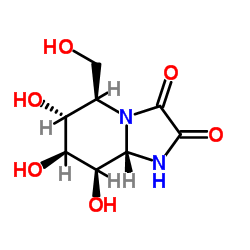 |
几夫碱
CAS:109944-15-2 |
C8H12N2O6 |
|
A context-independent N-glycan signal targets the misfolded ...
2014-12-15 [Biochem. J. 464(3) , 401-11, (2014)] |
|
Synthesis of kifunensine thioanalogs and their inhibitory ac...
2013-01-10 [Carbohydr. Res. 365 , 1-8, (2013)] |
|
Evolution of cross-neutralizing antibody specificities to th...
2012-01-01 [PLoS ONE 7(11) , e49610, (2012)] |
|
The unfolded protein response transducer ATF6 represents a n...
2013-11-01 [J. Biol. Chem. 288(44) , 31517-27, (2013)] |
|
Mannosidase I inhibition rescues the human alpha-sarcoglycan...
2008-05-01 [Hum. Mutat. 17 , 1214-21, (2008)] |