Effect of acidity on the enantiomeric resolution of thyroxine and tocainide by HPLC on a (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid column.
Hassan Y Aboul-Enein, Imran Ali, Myung Ho Hyun, Yoo Jae Cho, Jong Sun Jin
文献索引:J. Biochem. Biophys. Methods 54(1-3) , 407-13, (2002)
全文:HTML全文
摘要
Enantiomeric resolution of thyroxine and tocainide was achieved on a (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid column. The mobile phases were methanol/water (4:1, v/v) and methanol/water containing 5 mM sulfuric acid (4:1, v/v) for tocainide and thyroxine respectively. The flow rate was 0.5 ml/min. The effect of the acidity on the chiral resolution of these drugs was studied. Detection was at 220 nm for both drugs. The values of alpha and Rs were 2.08-3.11 and 1.00-2.60, respectively, for thyroxine while the values of alpha and Rs were 1.13-1.26 and 0.10-1.30, respectively, for tocainide.Copyright 2002 Elsevier Science B.V.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
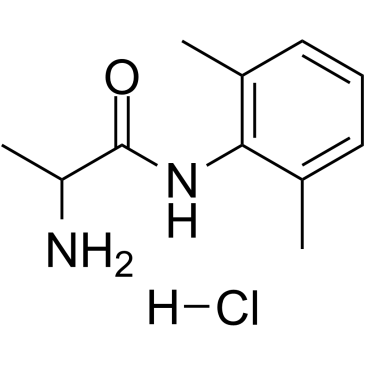 |
N-(2-氨基丙酰基)-2,6-二甲基苯胺
CAS:71395-14-7 |
C11H17ClN2O |
|
Synthesis, structure and pharmacology of acyl-2,6-xylidines.
2004-01-01 [Acta Pol. Pharm. 61(3) , 215-21, (2004)] |
|
Quantitative structure-pharmacokinetic relationships for dru...
2006-03-01 [J. Mol. Graph. Model. 24(5) , 383-95, (2006)] |
|
The efficacy of anticonvulsants on orofacial pain: a systema...
2011-05-01 [Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 111(5) , 627-33, (2011)] |
|
Non-antiepileptic drugs for trigeminal neuralgia.
2011-01-01 [Cochrane Database Syst. Rev. (1) , CD004029, (2011)] |
|
Tocainide analogues binding to human serum albumin: a HPLAC ...
2010-10-10 [J. Pharm. Biomed. Anal. 53(2) , 179-85, (2010)] |