Conformationally constrained histidines in the design of peptidomimetics: strategies for the χ-space control.
Azzurra Stefanucci, Francesco Pinnen, Federica Feliciani, Ivana Cacciatore, Gino Lucente, Adriano Mollica
文献索引:Int. J. Mol. Sci. 12 , 2853-2890, (2011)
全文:HTML全文
摘要
A successful design of peptidomimetics must come to terms with χ-space control. The incorporation of χ-space constrained amino acids into bioactive peptides renders the χ(1) and χ(2) torsional angles of pharmacophore amino acids critical for activity and selectivity as with other relevant structural features of the template. This review describes histidine analogues characterized by replacement of native α and/or β-hydrogen atoms with alkyl substituents as well as analogues with α, β-didehydro unsaturation or C(α)-C(β) cyclopropane insertion (ACC derivatives). Attention is also dedicated to the relevant field of β-aminoacid chemistry by describing the synthesis of β(2)- and β(3)-models (β-hHis). Structural modifications leading to cyclic imino derivatives such as spinacine, aza-histidine and analogues with shortening or elongation of the native side chain (nor-histidine and homo-histidine, respectively) are also described. Examples of the use of the described analogues to replace native histidine in bioactive peptides are also given.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
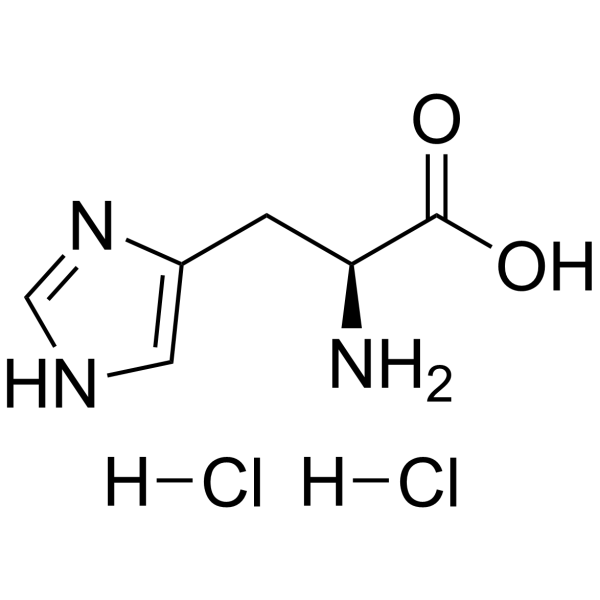 |
Histidine
CAS:6027-02-7 |
C6H10ClN3O2 |
|
Highly specific in vivo gene delivery for p53-mediated apopt...
2015-01-01 [Nat. Commun. 6 , 6456, (2015)] |
|
Histidine kinases and response regulators in networks.
2012-04-01 [Curr. Opin. Microbiol. 15(2) , 118-24, (2011)] |
|
Assessing the fractions of tautomeric forms of the imidazole...
2011-04-05 [Proc. Natl. Acad. Sci. U. S. A. 108 , 5602-5607, (2011)] |
|
Could carnosine or related structures suppress Alzheimer's d...
2007-05-01 [J. Alzheimers Dis. 11 , 229-240, (2007)] |
|
Influence of amino acid relative position on the oxidative m...
2011-03-01 [Anal. Bioanal. Chem 399 , 2779-2794, (2011)] |