Selective iron-catalyzed cross-coupling reactions of grignard reagents with enol triflates, acid chlorides, and dichloroarenes.
Bodo Scheiper, Melanie Bonnekessel, Helga Krause, Alois Fürstner
文献索引:J. Org. Chem. 69 , 3943-3949, (2004)
全文:HTML全文
摘要
Cheap, readily available, air stable, nontoxic, and environmentally benign iron salts such as Fe(acac)(3) are excellent precatalysts for the cross-coupling of Grignard reagents with alkenyl triflates and acid chlorides. Moreover, it is shown that dichloroarene and -heteroarene derivatives as the substrates can be selectively monoalkylated by this method. All cross-coupling reactions proceed very rapidly under notably mild conditions and turned out to be compatible with a variety of functional groups in both reaction partners. A detailed analysis of the preparative results suggests that iron-catalyzed C-C bond formations can occur via different pathways. Thus, it is likely that reactions of methylmagnesium halides involve iron-ate complexes as the active components, whereas reactions of Grignard reagents with two or more carbon atoms are effected by highly reduced iron-clusters of the formal composition [Fe(MgX)(2)](n) generated in situ. Control experiments using the ate-complex [Me(4)Fe]Li(2) corroborate this interpretation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
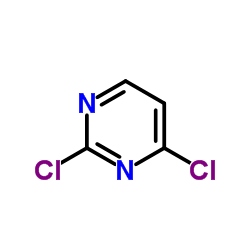 |
2,4-二氯嘧啶
CAS:3934-20-1 |
C4H2Cl2N2 |
|
Novel vanilloid receptor-1 antagonists: 1. Conformationally ...
2007-07-26 [J. Med. Chem. 50 , 3497, (2007)] |
|
One-pot Double Suzuki Couplings of Dichloropyrimidines.
2012-02-01 [Synthesis 2010(16) , 2721-2724, (2010)] |
|
An efficient route to 4-aryl-5-pyrimidinylimidazoles via seq...
2006-01-19 [Org. Lett. 8(2) , 269-72, (2006)] |
|
Chemistry-based risk assessment for skin sensitization: quan...
2011-07-18 [Chem. Res. Toxicol. 24(7) , 1003-11, (2011)] |
|
Synthesis and SAR of aminopyrimidines as novel c-Jun N-termi...
2007-06-15 [Bioorg. Med. Chem. Lett. 17 , 3463, (2007)] |