2,4-二氯嘧啶
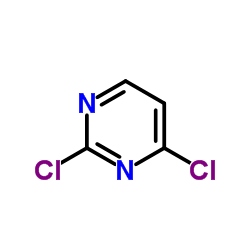
2,4-二氯嘧啶结构式

|
常用名 | 2,4-二氯嘧啶 | 英文名 | 2,4-Dichloropyrimidine |
|---|---|---|---|---|
| CAS号 | 3934-20-1 | 分子量 | 148.978 | |
| 密度 | 1.5±0.1 g/cm3 | 沸点 | 209.1±0.0 °C at 760 mmHg | |
| 分子式 | C4H2Cl2N2 | 熔点 | 57-61 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 104.3±5.4 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Novel vanilloid receptor-1 antagonists: 1. Conformationally restricted analogues of trans-cinnamides.
J. Med. Chem. 50 , 3497, (2007) The vanilloid receptor-1 (VR1 or TRPV1) is a member of the transient receptor potential (TRP) family of ion channels and plays a role as an integrator of multiple pain-producing stimuli. From a high-throughput screening assay, measuring calcium uptake in TRPV... |
|
|
One-pot Double Suzuki Couplings of Dichloropyrimidines.
Synthesis 2010(16) , 2721-2724, (2010) An effective one-pot, regioselective double Suzuki coupling of 2,4-dichloropyrimidine has been developed, which enables the quick and efficient synthesis of diarylated pyrimidines. The choice of solvent proved critical to the success of this reaction sequence... |
|
|
An efficient route to 4-aryl-5-pyrimidinylimidazoles via sequential functionalization of 2,4-dichloropyrimidine.
Org. Lett. 8(2) , 269-72, (2006) [reaction: see text] Starting from 2,4-dichloropyrimidine, a concise synthetic route to medicinally important 4-aryl-5-pyrimidinylimidazoles is described. Sequential substitution of the 4- and 2-chloro groups using a regioselective Sonogashira coupling, follo... |
|
|
Chemistry-based risk assessment for skin sensitization: quantitative mechanistic modeling for the S(N)Ar domain.
Chem. Res. Toxicol. 24(7) , 1003-11, (2011) There is a strong impetus to develop nonanimal based methods to predict skin sensitization potency. An approach based on physical organic chemistry, whereby chemicals are classified into reaction mechanistic domains and quantitative models or read-across meth... |
|
|
Selective iron-catalyzed cross-coupling reactions of grignard reagents with enol triflates, acid chlorides, and dichloroarenes.
J. Org. Chem. 69 , 3943-3949, (2004) Cheap, readily available, air stable, nontoxic, and environmentally benign iron salts such as Fe(acac)(3) are excellent precatalysts for the cross-coupling of Grignard reagents with alkenyl triflates and acid chlorides. Moreover, it is shown that dichloroaren... |
|
|
Synthesis and SAR of aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors.
Bioorg. Med. Chem. Lett. 17 , 3463, (2007) The development of a series of novel aminopyrimidines as inhibitors of c-Jun N-terminal kinases is described. The synthesis, in vitro inhibitory values for JNK1, JNK2 and CDK2, and the in vitro inhibitory value for a c-Jun cellular assay are discussed. |
|
|
Design, synthesis, and SAR of 2-dialkylamino-4-arylpyrimidines as potent and selective corticotropin-releasing factor(1) (CRF(1)) receptor antagonists.
Bioorg. Med. Chem. Lett. 14 , 2083-2086, (2004) A series of 2-dialkylamino-4-phenylpyrimidines (7) was designed and synthesized as CRF(1) antagonists. SAR studies of this series resulted in the discovery of potent and selective antagonists 7b and 7n bearing a 4-(2,4,6-trisubstituted-phenyl) ring and a bulk... |