Contractile activities of structural analogs of leukotrienes C and D: necessity of a hydrophobic region.
J M Drazen, R A Lewis, K F Austen, M Toda, F Brion, A Marfat, E J Corey
文献索引:Proc. Natl. Acad. Sci. U. S. A. 78 , 3195, (1981)
全文:HTML全文
摘要
Sixteen structural analogs of leukotrienes C and D were tested for their contractile activities on guinea pig pulmonary parenchymal strip and ileum. The analogs differed from the native structures in the position of either the thioether-linked peptide side chain or the hydroxyl group (or both) or in the number and positions of ethylenic bonds. Analogs in which the thioether-linked peptide chain was attached other than at the C-6 position had substantial reductions in activity on both smooth muscle preparations, whereas analogs in which the various ethylenic bonds were saturated retained substantial contractile activity in both assays. These observations demonstrate that, although a hydrophobic region of the eicosinoid is necessary for contractile activity, the length of this segment is more critical than its precise stereochemistry. Analogs of leukotrienes based on the possibility of parallel biosynthetic routes deriving from 8-, 11-, and 15-hydroperoxyeicosatetraenoic acid as precursors were found to effect a comparatively weak contractile response so that their role as biological agents in this respect seems unlikely.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
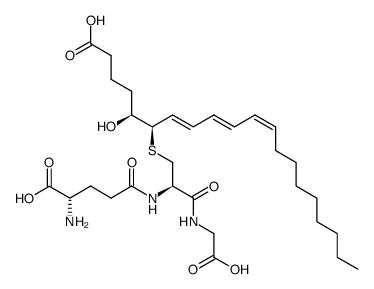 |
白三烯C3
CAS:77209-77-9 |
C30H49N3O9S |
|
Conversion of 5,8,11-eicosatrienoic acid to leukotrienes C3 ...
1981-03-10 [J. Biol. Chem. 256 , 2275, (1981)] |
|
Distribution and metabolism of 3H-labeled leukotriene C3 in ...
1982-01-10 [J. Biol. Chem. 257(1) , 531-5, (1982)] |
|
Metabolism of leukotriene C3 in the guinea pig. Identificati...
1981-09-25 [J. Biol. Chem. 256(18) , 9573-8, (1981)] |
|
Contractile activities of several cysteine-containing leukot...
2009-01-01 [Eur. J. Pharmacol. 86(2) , 207-15, (1982)] |
|
Metabolism of leukotriene C3.
1982-01-01 [Adv. Prostaglandin. Thromboxane. Leukot. Res. 9 , 83-101, (1982)] |