Change of substrate specificity by chemical modification of lysine residues of porcine pancreatic alpha-amylase.
H Yamashita, H Nakatani, B Tonomura
文献索引:Biochim. Biophys. Acta 1202(1) , 129-34, (1993)
全文:HTML全文
摘要
Lysine residues of porcine pancreatic alpha-amylase (PPA) were modified with trinitrobenzenesulfonate (TNBS). 6 out of 21 lysine residues were modified per PPA molecule. Amylase activity (hydrolysis of the alpha-1,4-D-glucoside bond) was decreased to about 50% of the native enzyme, as judged from the kcat value at pH 6.9 after the modification, whereas maltosidase activity (hydrolysis of p-nitrophenyl-alpha-D-maltoside producing p-nitrophenol and maltose) was increased to about 250%. The increase in maltosidase activity of the modified PPA was due to the increase in kcat, but not to the decrease in Km. Modification of PPA with five kinds of acid anhydrides also caused the same effect as TNBS, including the number of modified lysine residues. The degree of increase in maltosidase activity was fairly proportional to the volume of the incorporated modification reagent. A modification protection study in the presence of maltotriitol (G3OH), which protected two out of six modifiable lysine residues against modification, suggested that a lysine residue at the substrate-binding site contributes to the change of substrate specificity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
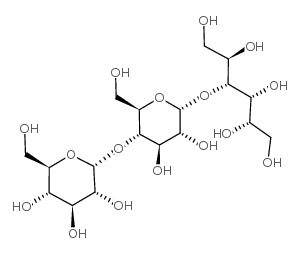 |
麦芽三糖醇
CAS:32860-62-1 |
C18H34O16 |
|
Inhibition and binding modes of low-molecular-weight inhibit...
1992-02-01 [J. Biochem. 111(2) , 182-5, (1992)] |
|
Inhibition of human digestive enzymes by hydrogenated malto-...
[Int. J. Vit. Nutr. Res. 51 , 161-5, (1981)] |
|
Maltitol and maltotriitol as inhibitors of acid production i...
1982-01-01 [Caries Res. 16 , 90-5, (1982)] |
|
Orientated growth of crystalline anhydrous maltitol (4-O-alp...
2004-04-28 [Carbohydr. Res. 339 , 1225-31, (2004)] |
|
Effect of maltotriitol on the action pattern of porcine panc...
1990-09-30 [Carbohydr. Res. 206(1) , 161-6, (1990)] |