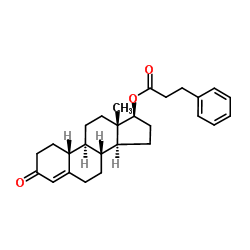
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KG7995000
-
CHEMICAL NAME :
-
Estr-4-en-3-one, 17-beta-hydroxy-, hydrocinnamate
-
CAS REGISTRY NUMBER :
-
62-90-8
-
BEILSTEIN REFERENCE NO. :
-
3170861
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C27-H34-O3
-
MOLECULAR WEIGHT :
-
406.61
-
WISWESSER LINE NOTATION :
-
L E5 B666 OV MUTJ E1 FOV2R
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
595 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 20,143,1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,541,1982 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
14850 ug/kg
-
SEX/DURATION :
-
male 11 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 26,1673,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
STEDAM Steroids. (Holden-Day Inc., 4432 Telegraph Ave., Oakland, CA 94609) V.1- 1963- Volume(issue)/page/year: 18,731,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 17-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
AVBIB9 Advances in the Biosciences. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1- 1967- Volume(issue)/page/year: 13,71,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
1400 ug/kg
-
SEX/DURATION :
-
male 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 23,907,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
3750 ug/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,723,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
15 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,723,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1200 ug/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 23,907,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
15 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,723,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
15 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,723,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,723,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
240 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,723,1973 *** REVIEWS *** TOXICOLOGY REVIEW BIREBV Biology of Reproduction. (Soc. for the Study of Reproduction, 309 W. Clark St., Champaign, IL 61820) V.1- 1969- Volume(issue)/page/year: 8,259,1973 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X5317 No. of Facilities: 7 (estimated) No. of Industries: 1 No. of Occupations: 2 No. of Employees: 628 (estimated) No. of Female Employees: 314 (estimated)
|