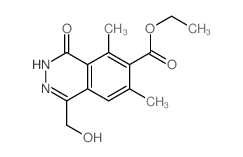
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TH8849440
-
CHEMICAL NAME :
-
6-Phthalazinecarboxylic acid, 3,4-dihydro-1-(hydroxymethyl)-5,7-dimethyl-4-oxo-, ethyl ester
-
CAS REGISTRY NUMBER :
-
56611-65-5
-
BEILSTEIN REFERENCE NO. :
-
0811823
-
LAST UPDATED :
-
199703
-
DATA ITEMS CITED :
-
16
-
MOLECULAR FORMULA :
-
C14-H16-N2-O4
-
MOLECULAR WEIGHT :
-
276.32
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1750 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6247,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
570 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6247,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1850 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6247,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
780 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6247,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1020 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6247,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6247,1982 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
134 gm/kg/13W-I
-
TOXIC EFFECTS :
-
Liver - other changes Endocrine - other changes Blood - changes in spleen
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6251,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
153600 mg/kg/32W-I
-
TOXIC EFFECTS :
-
Liver - other changes Kidney, Ureter, Bladder - other changes Blood - changes in spleen
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6275,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
73 gm/kg/1Y-I
-
TOXIC EFFECTS :
-
Cardiac - pulse rate increase, without fall in BP Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases Related to Chronic Data - death
-
REFERENCE :
-
TOXID9 Toxicologist. (Soc. of Toxicology, Inc., 475 Wolf Ledge Parkway, Akron, OH 44311) V.1- 1981- Volume(issue)/page/year: 6,202,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
31200 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Cardiac - other changes Cardiac - changes in heart weight
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6291,1982 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6365,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Specific Developmental Abnormalities - homeostasis
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6365,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6365,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
multigenerations
-
TOXIC EFFECTS :
-
Reproductive - Fertility - mating performance (e.g. # sperm positive females per # females mated; # copulations per # estrus cycles)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6365,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2600 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6389,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5200 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 16,6389,1982
|