CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
BW2275000
-
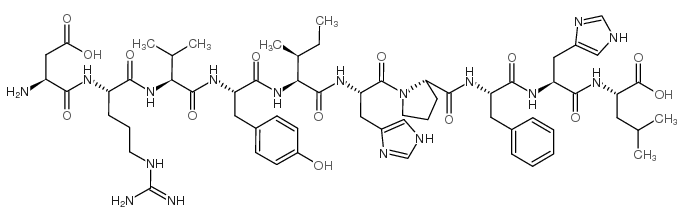
CHEMICAL NAME :
-
Angiotonin
-
CAS REGISTRY NUMBER :
-
1407-47-2
-
LAST UPDATED :
-
199012
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
8 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZIAQ "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973 Volume(issue)/page/year: -,43,1973 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
JPMSAE Journal of Pharmaceutical Sciences. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.50- 1961- Volume(issue)/page/year: 58,406,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - body wall
-
REFERENCE :
-
LIFSAK Life Sciences. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1-8, 1962-69; V.14- 1974- Volume(issue)/page/year: 8,525,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - homeostasis Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
LIFSAK Life Sciences. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1-8, 1962-69; V.14- 1974- Volume(issue)/page/year: 8,525,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
LIFSAK Life Sciences. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1-8, 1962-69; V.14- 1974- Volume(issue)/page/year: 8,525,1969
|