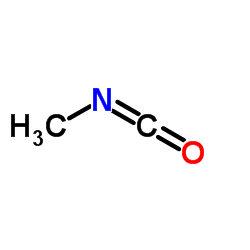
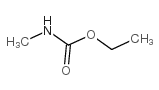
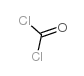
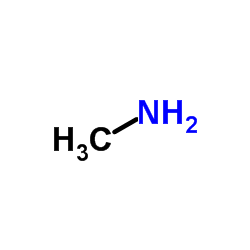
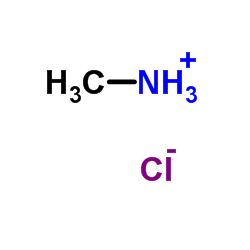
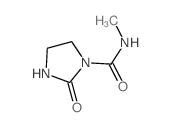
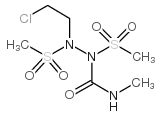
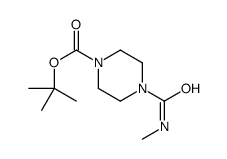
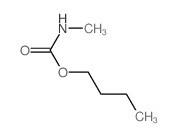
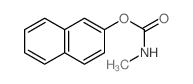
6452-47-7
| 中文名 | 甲胺基甲酰氯 |
|---|---|
| 英文名 | Methylaminoformyl Chloride |
| 中文别名 | 甲氨基甲酰氯 |
| 英文别名 |
EINECS 229-253-7
N-methylcarbamoyl chloride Methylcarbamic chloride Methylaminoformylchloride |
| 密度 | 1.2±0.1 g/cm3 |
|---|---|
| 沸点 | 93ºC (dec.) |
| 熔点 | 45ºC |
| 分子式 | C2H4ClNO |
| 分子量 | 93.512 |
| 精确质量 | 92.998138 |
| PSA | 29.10000 |
| LogP | 0.28 |
| 外观性状 | 白色晶体 |
| 折射率 | 1.416 |
| 储存条件 | 2-8℃ |
| 计算化学 | 1.疏水参数计算参考值(XlogP):0.6 2.氢键供体数量:1 3.氢键受体数量:1 4.可旋转化学键数量:0 5.互变异构体数量:2 6.拓扑分子极性表面积:29.1 7.重原子数量:5 8.表面电荷:0 9.复杂度:44.9 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Methylaminoformyl chloride Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. Section3. Composition/information on ingredients. Ingredient name:Methylaminoformyl chloride CAS number:6452-47-7 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Store in closed vessels, refrigerated. Storage: Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified Boiling point:No data No data Melting point: Flash point:No data Density:No data Molecular formula:C2H4ClNO Molecular weight:93.5 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
~56% 
6452-47-7 |
| 文献:D'Silva, Themistocles D. J.; Lopes, Anibal; Jones, Russell L.; Singhawangcha, Sureerat; Chan, John K. Journal of Organic Chemistry, 1986 , vol. 51, # 20 p. 3781 - 3788 |
|
~0% 
6452-47-7 |
| 文献:Mironov, V. F.; Kozyukov, V. P.; Orlov, G. I. J. Gen. Chem. USSR (Engl. Transl.), 1981 , vol. 51, # 8 p. 1814 - 1818,1555 - 1559 |
|
~% 
6452-47-7 |
| 文献:Synthesis, , # 10 art. no. Z03508SS, p. 1612 - 1618 |
|
~% 
6452-47-7 |
| 文献:US2480088 , ; |
|
~% 
6452-47-7 |
| 文献:Justus Liebigs Annalen der Chemie, , vol. 244, p. 35 |
| 上游产品 6 | |
|---|---|
| 下游产品 10 | |