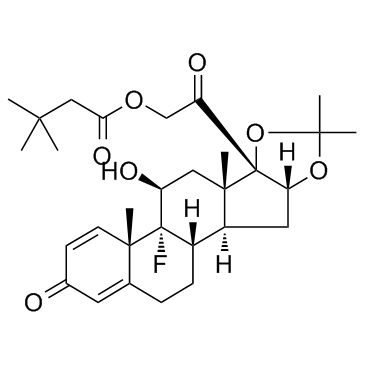
5611-51-8
| 中文名 | 己曲安奈德 |
|---|---|
| 英文名 | triamcinolone hexacetonide |
| 中文别名 | 曲安西龙叔丁乙酸酯 |
| 英文别名 |
Aristospan (TN)
Tiamcinoloni hexacetonidum [INN] EINECS 227-031-4 UNII-I7GT1U99Y9 TATBA Lederspan Triamcinolone esacetonide [DCIT] |
| 描述 | Triamcinolone hexacetonide是一种常用的长效类固醇,用于治疗亚急性和慢性炎性关节疾病。 |
|---|---|
| 相关类别 | |
| 体内研究 | 曲安奈德(hexamcinolone hexacetonide)在化学诱导的关节软骨损伤模型中产生显着的,剂量依赖性的保护作用。注射曲安奈德(hexamcinolone hexauttonide)的豚鼠显示出不太明显的原纤维形成和骨赘。细胞损失不太广泛。单次注射曲安奈德(triamcinolone hexacetonide)进入兔的同侧膝关节,经过部分外侧半月板切除术和横断骨间质和侧支腓骨韧带可减少软骨细胞克隆,细胞损失,骨赘形成和纤维性颤动[1]。商业上可获得的曲安奈德在玻璃体中的半衰期是曲安奈德己糖胺的两倍,但前者在该兔模型中对视网膜有毒。重新配制的异口服曲安奈德(triamcinolone hexacetonide)没有显示对视网膜功能或结构有害的证据[2]。曲安奈德在舌神经损伤部位的局部应用导致可能有益的变化,例如降低的机械敏感性和增强的再生[3]。 |
| 动物实验 | 豚鼠:给动物关节内注射0.1mL曲安奈德己酮,剂量为0.40mg / kg(组2和3)或0.04mg / kg(组4和5)。注射到第6组动物的膝盖中的CMC的体积与用于其他组中的关节内注射的CMC的体积相同,即0.1mL。当动物被杀死时,立即打开两个膝盖并进行严格检查[1]。 |
| 参考文献 |
| 密度 | 1.24 g/cm3 |
|---|---|
| 沸点 | 619.5ºC |
| 分子式 | C30H41FO7 |
| 分子量 | 532.64100 |
| 闪点 | 328.5ºC |
| 精确质量 | 532.28400 |
| PSA | 99.13000 |
| LogP | 4.40590 |
| 折射率 | 1.556 |
| 储存条件 | 库房通风低温干燥 |
|
Section 1. Chemical Product and Company Identification Triamcinolone Hexacetonide Common Name/ Trade Name Triamcinolone Hexacetonide Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at
least 15 minutes. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2), halogenated compounds. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various SubstancesNon-flammable in presence of shocks. Explosion Hazards in Presence Slightly explosive in presence of open flames and sparks. Non-explosive in presence of shocks. of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. As with most organic solids, fire is possible at elevated temperatures Special Remarks on Fire Hazards Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presences of an ignition source is a potential dust Hazardsexplosion hazard. Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Triamcinolone Hexacetonide Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Do not breathe dust. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Crystalline powder.)OdorOdorless. TasteNot available. Molecular Weight532.64 g/mole White. Off-white. Color Not available. pH (1% soln/water) Boiling PointNot available. Decomposition temperature: 295°C (563°F) Melting Point Critical TemperatureNot available. Specific GravityNot available. Vapor PressureNot applicable. Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Not available. Water/Oil Dist. Coeff. Ionicity (in Water)Not available. Dispersion PropertiesNot available. SolubilityInsoluble in cold water, hot water. Section 10. Stability and Reactivity Data StabilityThe product is stable. Not available. Instability Temperature Conditions of InstabilityNot available. Incompatibility with variousNot available. substances CorrosivityNot available. Triamcinolone Hexacetonide Not available. Special Remarks on Reactivity Not available. Special Remarks on Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans Not available. Other Toxic Effects on Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onMay cause adverse reproductive effects and birth defects (teratogenic) Human: passes through the placenta. Chronic Effects on Humans Special Remarks on otherPotential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: May cause eye irritation. Inhalation: May cause respiratory tract irritaition. Ingestion: May cause gastrointestinal tract irritation with abdominal distension, nausea, vomiting. May cause ulcerative esophagitis and peptic ulcers. May cause fluid and electrolyte disturbances resulting in sodium retention, fluid retention, congestive heart failure in susceptible individuals, potassium loss, hypokalemic alkalosis, increased calcium excretion, mild diuresis, polyuria, polydypsia, hypertension. May cause depression of appetite/anorexia in contrast to voracious appetite ordinarily encountered with other glucocorticoids. Common corticosteroids may cause euphoria whereas Triamcinolone may cause mood depression. May cause steroid myopathy/muscular atrophy with loss of muscle mass and muscle weakness involving muscles of the thighs, pelvis, and low back, muscle cramps. May cause other musculoskeletal effects such as Osteoporosis, vertebral conpression fractures, pathologic fractures of long bones. May affect immune system/immune response and increase the susceptibly to infections. May very rarely cause hypertension, hyperglycemia, and may affect behavior/central nervous system (excitation, vertigo, headache, convulsions, psychosis, hallucinations), and eye problems (glaucoma, mydriasis). Dermatologic effects may include impaired wound healing, thin fragile skin, facial erythema, petechiae and ecchymoses. Section 12. Ecological Information Not available. Ecotoxicity BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The products of degradation are more toxic than the product itself. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Triamcinolone Hexacetonide Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. Other ClassificationsWHMIS (Canada) Not controlled under WHMIS (Canada). DSCL (EEC)This product is not classified according Not applicable. to the EU regulations. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) Triamcinolone Hexacetonide ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 危险品运输编码 | NONH for all modes of transport |
|---|